Evaluation of Newborn Direct Bilirubin As Screening for Cholestatic Liver Disease
- PMID: 38034462
- PMCID: PMC10684158
- DOI: 10.1097/PG9.0000000000000345
Evaluation of Newborn Direct Bilirubin As Screening for Cholestatic Liver Disease
Abstract
Background: Biliary atresia (BA) remains the most common indication for pediatric liver transplantation. Early diagnosis is essential for a favorable long-term prognosis for patients with BA. Preliminary data suggests that measurement of direct bilirubin (DB) in newborns may be an effective screening tool for neonatal cholestasis, particularly BA, allowing for early referral and diagnosis. The objective of our study was to establish a cutoff DB value to predict diagnosis of cholestatic liver disease (CLD) with high sensitivity and specificity, as well as, to evaluate whether newborns with elevated DB received appropriate follow-up in our health system.
Methods: Baseline data were collected on infants born between 2016 and 2019 who had serum total bilirubin and DB drawn in the nursery, and who continued to follow in our health system. Sensitivity, specificity, and positive and negative predictive values were examined using cutoff values of 0.5, 0.6, and 0.7 mg/dL for identifying infants at risk for CLD. Patients' charts were reviewed to note whether they had follow-up levels drawn by their pediatrician or by the hepatology team within 2 months of age and whether they were diagnosed with CLD.
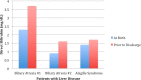
Results: Serum total bilirubin and DB levels were drawn from 11 965 infants during their hospitalizations. Three infants from this cohort were diagnosed with CLD: 2 with BA and 1 with Alagille syndrome. DB cutoff values of 0.5, 0.6, and 0.7 mg/dL had sensitivity of 100% and specificity of 96.83% (95% confidence interval [CI], 96.69%-97.53%), 99.08% (95% CI, 98.81%-99.30%), and 99.63% (95% CI, 99.4%-99.7%), respectively. Given that a DB of 0.6 mg/dL had a sensitivity of 100% and specificity of 99%, this value was chosen as the cutoff value to monitor for DB follow-up and diagnosis of CLD. Out of 60 infants who met criteria for DB ≥0.6 mg/dL, only 15 (25%) had a repeat level drawn after nursery discharge; 3 (5%) were eventually diagnosed with CLD.
Conclusions: A DB cutoff value of 0.6 mg/dL yielded high sensitivity and specificity for identifying patients with CLD. All 3 patients diagnosed with CLD had elevated DB at hospital discharge. The data revealed that the majority (75%) of eligible newborns did not receive follow-up for their elevated DB in the outpatient setting.
Keywords: biliary atresia; direct hyperbilirubinemia; liver transplantation.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:154–168. - PubMed
-
- Schreiber RA, Barker CC, Roberts EA, et al. ; Canadian Pediatric Hepatology Research Group. Biliary atresia: the Canadian experience. J Pediatr. 2007;151:659–665, 665.e1. - PubMed
-
- Madsen SS, Kvist N, Thorup J. Increased conjugated bilirubin is sufficient to initiate screening for biliary atresia. Dan Med J. 2015;62:A5114. - PubMed
-
- Matsui A. Screening for biliary atresia. Pediatr Surg Int. 2017;33:1305–1313. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous