The inhibitory effect of trastuzumab on BT474 triple‑positive breast cancer cell viability is reversed by the combination of progesterone and estradiol
- PMID: 38034484
- PMCID: PMC10688505
- DOI: 10.3892/ol.2023.14152
The inhibitory effect of trastuzumab on BT474 triple‑positive breast cancer cell viability is reversed by the combination of progesterone and estradiol
Abstract
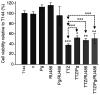
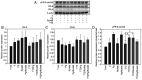
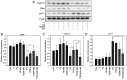
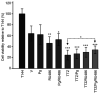
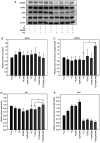
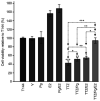
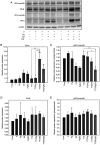
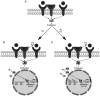
Breast cancer expressing the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) is known as triple-positive (TPBC). TPBC represents 9-11% of breast cancer cases worldwide and is a heterogeneous subtype. Notably, TPBC presents a therapeutic challenge due to the crosstalk between the hormonal (ER and PR) and HER2 pathways. Patients with TPBC are treated with trastuzumab (TTZ); however, several patients treated with TTZ tend to relapse. The present study aimed to investigate the effect of the PR on inhibitory effect of TTZ on cell viability. BT474 cells (a model of TPBC) and BT474 PR-silenced cells were treated with either TTZ, progesterone (Pg), the PR antagonist mifepristone (RU486) or estradiol (E2) alone or in combination for 144 h (6 days). Cell viability assays and western blotting were subsequently performed. The results showed that Pg and E2 interfered with the inhibitory effect of TTZ on cell viability and this effect was potentiated when both hormones were combined. Pg was revealed to act through the PR, mainly activating the PR isoform B (PR-B) and inducing the protein expression levels of CDK4 and cyclin D1; however, it did not reactivate the HER2/Akt pathway. By contrast, E2 was able to increase PR isoform A (PR-A) expression, which was inhibited by Pg. Notably, in most of the experiments, RU486 did not antagonize the effects of Pg. In conclusion, Pg and E2 may interfere with the inhibitory effect of TTZ on cell viability through PR-B activation and PR-A inactivation.
Keywords: estradiol; progesterone; trastuzumab; triple-positive breast cancer.
Copyright: © López-Méndez et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
