Safety and efficacy of canakinumab treatment for undifferentiated autoinflammatory diseases: the data of a retrospective cohort two-centered study
- PMID: 38034538
- PMCID: PMC10685903
- DOI: 10.3389/fmed.2023.1257045
Safety and efficacy of canakinumab treatment for undifferentiated autoinflammatory diseases: the data of a retrospective cohort two-centered study
Abstract
Introduction: The blockade of interleukine-1 (anakinra and canakinumab) is a well-known highly effective tool for monogenic autoinflammatory diseases (AIDs), such as familial Mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, hyperimmunoglobulinaemia D syndrome, and cryopyrin-associated periodic syndrome, but this treatment has not been assessed for patients with undifferentiated AIDs (uAIDs). Our study aimed to assess the safety and efficacy of canakinumab for patients with uAIDs.
Methods: Information on 32 patients with uAIDs was retrospectively collected and analyzed. Next-generation sequencing and Federici criteria were used for the exclusion of the known monogenic AID.
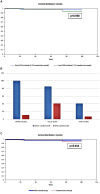
Results: The median age of the first episode was 2.5 years (IQR: 1.3; 5.5), that of the disease diagnosis was 5.7 years (IQR: 2.5;12.7), and that of diagnostic delay was 1.1 years (IQR: 0.4; 6.1). Patients had variations in the following genes: IL10, NLRP12, STAT2, C8B, LPIN2, NLRC4, PSMB8, PRF1, CARD14, IFIH1, LYST, NFAT5, PLCG2, COPA, IL23R, STXBP2, IL36RN, JAK1, DDX58, LACC1, LRBA, TNFRSF11A, PTHR1, STAT4, TNFRSF1B, TNFAIP3, TREX1, and SLC7A7. The main clinical features were fever (100%), rash (91%; maculopapular predominantly), joint involvement (72%), splenomegaly (66%), hepatomegaly (59%), lymphadenopathy (50%), myalgia (28%), heart involvement (31%), intestinal involvement (19%); eye involvement (9%), pleuritis (16%), ascites (6%), deafness, hydrocephalia (3%), and failure to thrive (25%). Initial treatment before canakinumab consisted of non-biologic therapies: non-steroidal anti-inflammatory drugs (NSAID) (91%), corticosteroids (88%), methotrexate (38%), intravenous immunoglobulin (IVIG) (34%), cyclosporine A (25%), colchicine (6%) cyclophosphamide (6%), sulfasalazine (3%), mycophenolate mofetil (3%), hydroxychloroquine (3%), and biologic drugs: tocilizumab (62%), sarilumab, etanercept, adalimumab, rituximab, and infliximab (all 3%). Canakinumab induced complete remission in 27 patients (84%) and partial remission in one patient (3%). Two patients (6%) were primary non-responders, and two patients (6%) further developed secondary inefficacy. All patients with partial efficacy or inefficacy were switched to tocilizumab (n = 4) and sarilumab (n = 1). The total duration of canakinumab treatment was 3.6 (0.1; 8.7) years. During the study, there were no reported Serious Adverse Events (SAEs). The patients experienced non-frequent mild respiratory infections at a rate that is similar as before canakinumab is administered. Additionally, one patient developed leucopenia, but it was not necessary to stop canakinumab for this patient.
Conclusion: The treatment of patients with uAIDs using canakinumab was safe and effective. Further randomized clinical trials are required to confirm the efficacy and safety.
Keywords: AID; autoinflammation; autoinflammatory disorders; canakinumab; interleukin-1; undifferentiated autoinflammatory disorders.
Copyright © 2023 Alexeeva, Shingarova, Dvoryakovskaya, Lomakina, Fetisova, Isaeva, Chomakhidze, Chibisova, Krekhova, Kozodaeva, Savostyanov, Pushkov, Zhanin, Demyanov, Suspitsin, Belozerov and Kostik.
Conflict of interest statement
EA received research grants from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Sanofi, Amgen, and Novartis, speaker at the Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer bureau. TD received research grants from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Amgen, and Novartis, speaker at the Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer bureau. KI received research grants from Roche, Novartis, and Sanofi. OL received research grants from Pfizer and Eli Lilly. EK speaker at the Novartis bureau. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, et al. . Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther. (2011) 13:R34. 10.1186/ar3266 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

