Efficacy of Chinese Herbal Medicine Formula in the Treatment of Mild to Moderate Erectile Dysfunction: Study Protocol for a Multi-Center, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial
- PMID: 38034900
- PMCID: PMC10683653
- DOI: 10.2147/IJGM.S436347
Efficacy of Chinese Herbal Medicine Formula in the Treatment of Mild to Moderate Erectile Dysfunction: Study Protocol for a Multi-Center, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial
Abstract
Introduction: Erectile dysfunction (ED) is a prevalent condition in urology, primarily managed with PDE5 inhibitors (PDE5Is). However, approximately 20% of patients do not experience improvement in overall sexual satisfaction (OS) after taking PDE5Is. Among these, traditional Chinese medicine (TCM) has emerged as a complementary approach, with formulas like Hongjing I granules (HJIG) showing promise in preliminary studies. This study aims to rigorously evaluate the effectiveness and safety of HJIG in mild to moderate ED cases, assessing improvement in both sexual function and TCM pattern alignment.
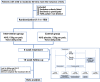
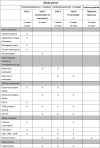
Methods: This study is a randomized, double-blind, placebo-controlled multicentre trial. Recruitment will be conducted from patients who have a strong willingness to try using only traditional Chinese medicine treatment (This is very common in traditional Chinese medicine hospitals.). A total of 100 patients diagnosed with mild to moderate ED caused by qi deficiency and blood stasis will be recruited and randomly assigned to receive one of two treatments: HJIG (N = 50) or placebo (N = 50). Patients will receive 8 weeks of treatment and a 16-week follow-up starting from the fourth week of treatment. Outcome measures, including the International Index of Erectile Function-Erectile Function domain (IIEF-EF) score, Sexual Encounter Profile (SEP), and Traditional Chinese Medicine symptom score, will be evaluated.
Discussion: The expected outcome of this trial is that the use of the herbal formula HIJG alone can improve overall sexual satisfaction (OS) in patients with mild to moderate ED, while also improving their traditional Chinese medicine symptom scores. This will provide evidence-based support for the use of Chinese medicine in the treatment of ED in China.
Trial registration: Chinese Clinical Trial Registry, ChiCTR2000041127, Registered on 19 December 2020, https://www.chictr.org.cn/showproj.html?proj=46469.
Trial status: Recruitment began in March 2021, therefore 80 patients have been recruited. It is expected to finish recruiting in December 2023.
Keywords: Chinese herbal formula; Hongjing I granules; alternative medicine; clinical protocols; clinical trials; erectile dysfunction; traditional Chinese medicine.
© 2023 Xu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Efficacy of Hongjing I granule, an herbal medicine, in patients with mild to moderate erectile dysfunction in a randomized controlled trial.Front Pharmacol. 2024 Dec 24;15:1367812. doi: 10.3389/fphar.2024.1367812. eCollection 2024. Front Pharmacol. 2024. PMID: 39776582 Free PMC article.
-
The efficacy and safety of Chinese herbal medicine Shen-Qi Hua-Yu formula in patients with diabetic lower extremity artery disease: Study protocol of a multi-center, randomized, double-blind, placebo-controlled trial.Medicine (Baltimore). 2020 Jan;99(3):e18713. doi: 10.1097/MD.0000000000018713. Medicine (Baltimore). 2020. PMID: 32011447 Free PMC article.
-
Traditional Chinese medicine syndrome differentiation and treatment by stages of Parkinson's disease: study protocol for a multicentre, randomized, double-blind, placebo-controlled clinical trial.Chin Med. 2022 Jun 13;17(1):68. doi: 10.1186/s13020-022-00625-4. Chin Med. 2022. PMID: 35698234 Free PMC article.
-
Chinese herbal medicine combined with tadalafil for erectile dysfunction: a systematic review and meta-analysis.Andrology. 2020 Mar;8(2):268-276. doi: 10.1111/andr.12696. Epub 2019 Aug 28. Andrology. 2020. PMID: 31464074
-
Spotlight on vardenafil in erectile dysfunction.Drugs Aging. 2004;21(2):135-40. doi: 10.2165/00002512-200421020-00005. Drugs Aging. 2004. PMID: 14960129 Review.
Cited by
-
Efficacy of Chinese herbal medicine in the treatment of anxiety and depression in male sexual dysfunction: a systematic review and meta-analysis protocol.Front Psychiatry. 2025 May 2;16:1584306. doi: 10.3389/fpsyt.2025.1584306. eCollection 2025. Front Psychiatry. 2025. PMID: 40386113 Free PMC article.
-
Analysis of vacuum negative pressure therapy and traditional Chinese medicine lavage in combination with tadalafil for vascular erectile dysfunction.Front Reprod Health. 2024 Feb 5;6:1335239. doi: 10.3389/frph.2024.1335239. eCollection 2024. Front Reprod Health. 2024. PMID: 38375500 Free PMC article. Review.
-
Efficacy of Hongjing I granule, an herbal medicine, in patients with mild to moderate erectile dysfunction in a randomized controlled trial.Front Pharmacol. 2024 Dec 24;15:1367812. doi: 10.3389/fphar.2024.1367812. eCollection 2024. Front Pharmacol. 2024. PMID: 39776582 Free PMC article.
-
Efficacy of Chinese herbal medicine in the treatment of anxiety and depression in male sexual dysfunction: a systematic review and meta-analysis.Sex Med. 2025 Jul 10;13(3):qfaf048. doi: 10.1093/sexmed/qfaf048. eCollection 2025 Jun. Sex Med. 2025. PMID: 40641608 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

