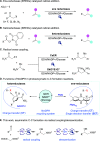
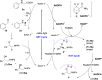
Photoredox/Enzymatic Catalysis Enabling Redox-Neutral Decarboxylative Asymmetric C-C Coupling for Asymmetric Synthesis of Chiral 1,2-Amino Alcohols
- PMID: 38034963
- PMCID: PMC10685423
- DOI: 10.1021/jacsau.3c00366
Photoredox/Enzymatic Catalysis Enabling Redox-Neutral Decarboxylative Asymmetric C-C Coupling for Asymmetric Synthesis of Chiral 1,2-Amino Alcohols
Abstract
Photocatalysis offers tremendous opportunities for enzymes to access new functions. Herein, we described a redox-neutral photocatalysis/enzymatic catalysis system for the asymmetric synthesis of chiral 1,2-amino alcohols via decarboxylative radical C-C coupling of N-arylglycines and aldehydes by combining an organic photocatalyst, eosin Y, and carbonyl reductase RasADH. Notably, this protocol avoids using any sacrificial reductants. A possible reaction mechanism proposed is that the transformation proceeds through sequential photoinduced decarboxylative radical addition to an aldehyde and a photoenzymatic deracemization pathway. This redox-neutral photoredox/enzymatic strategy is promising not only for effective synthesis of a series of chiral amino alcohols in a green and sustainable manner but also for the design of other novel C-C radical coupling transformations for the synthesis of bioactive molecules.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Enantioselective Alkyl-Acyl Radical Cross-Coupling Enabled by Metallaphotoredox Catalysis.J Am Chem Soc. 2025 Apr 2;147(13):10999-11009. doi: 10.1021/jacs.4c15275. Epub 2025 Feb 19. J Am Chem Soc. 2025. PMID: 39968896
-
Synthesis of 1,2-Amino Alcohols by Photoredox-Mediated Decarboxylative Coupling of α-Amino Acids and DNA-Conjugated Carbonyls.Org Lett. 2020 Dec 18;22(24):9484-9489. doi: 10.1021/acs.orglett.0c03461. Epub 2020 Nov 10. Org Lett. 2020. PMID: 33170713
-
Deracemization through Sequential Photoredox-Neutral and Chiral Brønsted Acid Catalysis.Angew Chem Int Ed Engl. 2022 Dec 5;61(49):e202211241. doi: 10.1002/anie.202211241. Epub 2022 Nov 9. Angew Chem Int Ed Engl. 2022. PMID: 36250910
-
Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1'-bi-2-naphthols (BINOLs).Acc Chem Res. 2014 May 20;47(5):1523-35. doi: 10.1021/ar500020k. Epub 2014 Apr 16. Acc Chem Res. 2014. PMID: 24738985 Free PMC article. Review.
-
Bridging chemistry and biology for light-driven new-to-nature enantioselective photoenzymatic catalysis.Chem Soc Rev. 2025 Jun 3;54(11):5157-5188. doi: 10.1039/d4cs00561a. Chem Soc Rev. 2025. PMID: 40351234 Review.
Cited by
-
Asymmetric Synthesis of 2-Arylethylamines: A Metal-Free Review of the New Millennium.Molecules. 2024 Dec 4;29(23):5729. doi: 10.3390/molecules29235729. Molecules. 2024. PMID: 39683888 Free PMC article. Review.
-
Ground-state flavin-dependent enzymes catalyzed enantioselective radical trifluoromethylation.Nat Commun. 2025 Jan 31;16(1):1225. doi: 10.1038/s41467-025-56437-1. Nat Commun. 2025. PMID: 39890795 Free PMC article.
-
Stereoselective Biosynthesis of Bichiral Amino Alcohols from Diketone via Difunctionalization.ACS Omega. 2025 May 27;10(22):23409-23420. doi: 10.1021/acsomega.5c01864. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521437 Free PMC article.
References
LinkOut - more resources
Full Text Sources