Parasitic infections related to anti-type 2 immunity monoclonal antibodies: a disproportionality analysis in the food and drug administration's adverse event reporting system (FAERS)
- PMID: 38035014
- PMCID: PMC10682182
- DOI: 10.3389/fphar.2023.1276340
Parasitic infections related to anti-type 2 immunity monoclonal antibodies: a disproportionality analysis in the food and drug administration's adverse event reporting system (FAERS)
Abstract
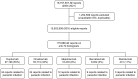
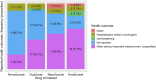
Introduction: Monoclonal antibodies (mAbs) targeting immunoglobulin E (IgE) [omalizumab], type 2 (T2) cytokine interleukin (IL) 5 [mepolizumab, reslizumab], IL-4 Receptor (R) α [dupilumab], and IL-5R [benralizumab]), improve quality of life in patients with T2-driven inflammatory diseases. However, there is a concern for an increased risk of helminth infections. The aim was to explore safety signals of parasitic infections for omalizumab, mepolizumab, reslizumab, dupilumab, and benralizumab. Methods: Spontaneous reports were used from the Food and Drug Administration's Adverse Event Reporting System (FAERS) database from 2004 to 2021. Parasitic infections were defined as any type of parasitic infection term obtained from the Standardised Medical Dictionary for Regulatory Activities® (MedDRA®). Safety signal strength was assessed by the Reporting Odds Ratio (ROR). Results: 15,502,908 reports were eligible for analysis. Amongst 175,888 reports for omalizumab, mepolizumab, reslizumab, dupilumab, and benralizumab, there were 79 reports on parasitic infections. Median age was 55 years (interquartile range 24-63 years) and 59.5% were female. Indications were known in 26 (32.9%) reports; 14 (53.8%) biologicals were reportedly prescribed for asthma, 8 (30.7%) for various types of dermatitis, and 2 (7.6%) for urticaria. A safety signal was observed for each biological, except for reslizumab (due to lack of power), with the strongest signal attributed to benralizumab (ROR = 15.7, 95% Confidence Interval: 8.4-29.3). Conclusion: Parasitic infections were disproportionately reported for mAbs targeting IgE, T2 cytokines, or T2 cytokine receptors. While the number of adverse event reports on parasitic infections in the database was relatively low, resulting safety signals were disproportionate and warrant further investigation.
Keywords: FAERS; biologicals; disproportionality analysis; helminth infections; monoclonal antibodies; parasitic infections; pharmacovigilance; spontaneous reporting.
Copyright © 2023 Pera, Brusselle, Riemann, Kors, Van Mulligen, Parry, de Wilde, Rijnbeek and Verhamme.
Conflict of interest statement
The research group from the Department of Medical Informatics receives/received unconditional research grants from Chiesi, GlaxoSmithKline (GSK), UCB, and Amgen, Johnson and Johnson, and the European Medicines Agency, none of which relate to the content of this manuscript. GB received payment/honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca, Boehringer-Ingelheim, Chiesi, GSK, Merck Sharp & Dohme (MSD), Novartis, and Sanofi, none of which relate to the content of this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

