Optimal combination of MYCN differential gene and cellular senescence gene predicts adverse outcomes in patients with neuroblastoma
- PMID: 38035110
- PMCID: PMC10687280
- DOI: 10.3389/fimmu.2023.1309138
Optimal combination of MYCN differential gene and cellular senescence gene predicts adverse outcomes in patients with neuroblastoma
Abstract
Introduction: Neuroblastoma (NB) is a common extracranial tumor in children and is highly heterogeneous. The factors influencing the prognosis of NB are not simple.
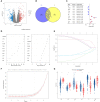
Methods: To investigate the effect of cell senescence on the prognosis of NB and tumor immune microenvironment, 498 samples of NB patients and 307 cellular senescence-related genes were used to construct a prediction signature.
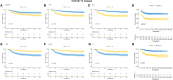
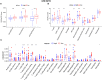
Results: A signature based on six optimal candidate genes (TP53, IL-7, PDGFRA, S100B, DLL3, and TP63) was successfully constructed and proved to have good prognostic ability. Through verification, the signature had more advantages than the gene expression level alone in evaluating prognosis was found. Further T cell phenotype analysis displayed that exhausted phenotype PD-1 and senescence-related phenotype CD244 were highly expressed in CD8+ T cell in MYCN-amplified group with higher risk-score.
Conclusion: A signature constructed the six MYCN-amplified differential genes and aging-related genes can be used to predict the prognosis of NB better than using each high-risk gene individually and to evaluate immunosuppressed and aging tumor microenvironment.
Keywords: COLD TUMOR; cellular senescence; neuroblastoma; prognosis; tumor microenvironment.
Copyright © 2023 Tan, Wang, Jin, Xia, Gong and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Transcript signatures that predict outcome and identify targetable pathways in MYCN-amplified neuroblastoma.Mol Oncol. 2016 Nov;10(9):1461-1472. doi: 10.1016/j.molonc.2016.07.012. Epub 2016 Aug 18. Mol Oncol. 2016. PMID: 27599694 Free PMC article.
-
Metronomic topotecan impedes tumor growth of MYCN-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence.Oncotarget. 2016 Jan 19;7(3):3571-86. doi: 10.18632/oncotarget.6527. Oncotarget. 2016. PMID: 26657295 Free PMC article.
-
Druggable epigenetic suppression of interferon-induced chemokine expression linked to MYCN amplification in neuroblastoma.J Immunother Cancer. 2021 May;9(5):e001335. doi: 10.1136/jitc-2020-001335. J Immunother Cancer. 2021. PMID: 34016720 Free PMC article.
-
The MYCN oncogene and differentiation in neuroblastoma.Semin Cancer Biol. 2011 Oct;21(4):256-66. doi: 10.1016/j.semcancer.2011.08.001. Epub 2011 Aug 9. Semin Cancer Biol. 2011. PMID: 21849159 Review.
-
Biological categories of neuroblastoma based on the international neuroblastoma pathology classification for treatment stratification.Pathol Int. 2021 Apr;71(4):232-244. doi: 10.1111/pin.13085. Epub 2021 Mar 3. Pathol Int. 2021. PMID: 33657257 Review.
Cited by
-
Cell Senescence-Related Genes as Biomarkers for Prognosis and Immunotherapeutic Response in Colon Cancer.Biochem Genet. 2025 Feb;63(1):124-143. doi: 10.1007/s10528-024-10690-z. Epub 2024 Feb 27. Biochem Genet. 2025. PMID: 38411939
-
Construction of a ferroptosis-based prediction model for the prognosis of MYCN-amplified neuroblastoma and screening and verification of target sites.Hereditas. 2025 Mar 19;162(1):41. doi: 10.1186/s41065-025-00413-8. Hereditas. 2025. PMID: 40108662 Free PMC article.
-
Targeting the ubiquitin-proteasome system: a novel therapeutic strategy for neuroblastoma.Front Oncol. 2024 Sep 26;14:1443256. doi: 10.3389/fonc.2024.1443256. eCollection 2024. Front Oncol. 2024. PMID: 39391247 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

