A new candidate epitope-based vaccine against PspA PhtD of Streptococcus pneumoniae: a computational experimental approach
- PMID: 38035337
- PMCID: PMC10684780
- DOI: 10.3389/fcimb.2023.1271143
A new candidate epitope-based vaccine against PspA PhtD of Streptococcus pneumoniae: a computational experimental approach
Abstract
Introduction: Pneumococcus is an important respiratory pathogen that is associated with high rates of death in newborn children and the elderly. Given the disadvantages of current polysaccharide-based vaccines, the most promising alternative for developing improved vaccines may be to use protein antigens with different roles in pneumococcus virulence. PspA and PhtD, highly immunogenic surface proteins expressed by almost all pneumococcal strains, are capable of eliciting protective immunity against lethal infections.
Methods: In this study using immunoinformatics approaches, we constructed one fusion construct (called PAD) by fusing the immunodominant regions of PspA from families 1 & 2 (PA) to the immunodominant regions of PhtD (PD). The objective of this project was to test the immunogenicity of the fusion protein PAD and to compare its protective activity against S. pneumoniae infection with PA or PD alone and a combination of PA and PD. The prediction of physicochemical properties, antigenicity, allergenicity, toxicity, and 3D-structure of the constructs, as well as molecular docking with HLA receptor and immune simulation were performed using computational tools. Finally, mice were immunized and the serum levels of antibodies/cytokines and functionality of antibodies in vitro were evaluated after immunization. The mice survival rates and decrease of bacterial loads in the blood/spleen were examined following the challenge.
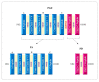
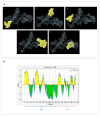
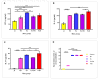
Results: The computational analyses indicated the proposed constructs could be antigenic, non-allergenic, non-toxic, soluble and able to elicit robust immune responses. The results of actual animal experiments revealed the candidate vaccines could induce the mice to produce high levels of antibodies and cytokines. The complement-mediated bactericidal activity of antibodies was confirmed and the antibodies provided favorable survival in immunized mice after bacterial challenge. In general, the experimental results verified the immunoinformatics studies.
Conclusion: For the first time this report presents novel peptide-based vaccine candidates consisting of immunodominant regions of PspA and PhtD antigens. The obtained findings confirmed that the fusion formulation could be relatively more efficient than the individual and combination formulations. The results propose that the fusion protein alone could be used as a serotype-independent pneumococcal vaccine or as an effective partner protein for a conjugate polysaccharide vaccine.
Keywords: Streptococcus pneumoniae; actual animal experiments; fusion protein; immunodominant B and T cell epitopes; immunoinformatics; pneumococcal epitope-based vaccine; pneumococcal histidine triad protein D (PhtD); pneumococcal surface protein A (PspA).
Copyright © 2023 Shafaghi, Bahadori, Barzi, Afshari, Madanchi, Mousavi and Shabani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Afshari E., Ahangari Cohan R., Shams Nosrati M. S., Mousavi S. F. (2023. a). Development of a bivalent protein-based vaccine against invasive pneumococcal diseases based on novel pneumococcal surface protein A in combination with pneumococcal histidine triad protein D. Front. Immunol. 14, 1187773. doi: 10.3389/fimmu.2023.1187773 - DOI - PMC - PubMed
-
- Afshari E., Cohan R. A., Sotoodehnejadnematalahi F., Mousavi S. F. (2023. b). In-silico design and evaluation of an epitope-based serotype-independent promising vaccine candidate for highly cross-reactive regions of pneumococcal surface protein A. J. Trans. Med. 21 (1), 13. doi: 10.1186/s12967-022-03864-z - DOI - PMC - PubMed
-
- Ahmadi K., Pouladfar G., Kalani M., Faezi S., Pourmand M. R., Hasanzadeh S., et al. . (2019). Epitope-based immunoinformatics study of a novel Hla-MntC-SACOL0723 fusion protein from Staphylococcus aureus: Induction of multi-pattern immune responses. Mol. Immunol. 114, 88–99. doi: 10.1016/j.molimm.2019.05.016 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

