Anticoagulation in Patients with Chronic Kidney Disease
- PMID: 38035566
- PMCID: PMC10994631
- DOI: 10.1159/000535546
Anticoagulation in Patients with Chronic Kidney Disease
Abstract
Background: Both atrial fibrillation and venous thromboembolism (VTE) are highly prevalent among patients with chronic kidney disease (CKD). Until recently, warfarin was the most commonly prescribed oral anticoagulant. Direct oral anticoagulants (DOACs) have important advantages and have been shown to be noninferior to warfarin with respect to stroke prevention or recurrent VTE in the general population, with lower bleeding rates. This review article will provide available evidence on the use of DOACs in patients with CKD.
Summary: In post hoc analyses of major randomized studies with DOACs for stroke prevention in atrial fibrillation, in the subgroup of participants with moderate CKD, defined as a creatinine clearance (CrCl) of 30-50 mL/min, dabigatran 150 mg and apixaban were associated with lower rates of stroke and systemic embolism, whereas apixaban and edoxaban were associated with lower bleeding and mortality rates, compared with warfarin. In retrospective observational studies in patients with advanced CKD (defined as a CrCl <30 mL/min) and atrial fibrillation, DOACs had similar efficacy with warfarin with numerically lower bleeding rates. All agents warrant dose adjustment in moderate-to-severe CKD. In patients on maintenance dialysis, the VALKYRIE trial, which was designed initially to study the effect of vitamin K on vascular calcification progression, established superiority for rivaroxaban compared with a vitamin K antagonist (VKA) in the extension phase. Two other clinical trials using apixaban (AXADIA and RENAL-AF) in this population were inconclusive due to recruitment challenges and low event rates. In post hoc analyses of randomized studies with DOACs in patients with VTE, in the subgroup of participants with moderate CKD at baseline, edoxaban was associated with lower rates of recurrent VTE, whereas rivaroxaban and dabigatran were associated with lower and higher bleeding rates, respectively, as compared to warfarin.
Key messages: DOACs have revolutionized the management of atrial fibrillation and VTE, and they should be preferred over warfarin in patients with moderate-to-severe CKD with appropriate dose adjustment. Therapeutic drug monitoring with a valid technique may be considered to guide clinical management in individualized cases. Current evidence questions the need for oral anticoagulation in patients on maintenance dialysis with atrial fibrillation as both DOACs and VKAs are associated with high rates of major bleeding.
Keywords: Atrial fibrillation; Chronic kidney disease; Direct oral anticoagulants; Pharmacokinetics; Venous thromboembolism; Warfarin.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Dr. Elenjickal received salary support from an educational grant by Pfizer. Dr. Travlos has nothing to disclose. Dr. Marques received salary support from an educational grant by Janssen. Dr. Mavrakanas received speaker honoraria from BMS Canada, Janssen, Astra Zeneca, and Pfizer and has served on advisory boards for Böhringer Ingelheim, Bayer, GSK, and Servier outside the submitted work. He has also received an unrestricted research grant from Astra Zeneca and operational grants from the Kidney Foundation of Canada, the Heart & Stroke foundation of Canada, and the Canadian Institute of Health Research. He is receiving salary support from the Fonds de Recherche Quebec Santé (Junior 1 Clinician Scholar Award # 298742) and is supported by a KRESCENT New Investigator Award.
Figures
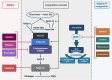
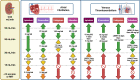
References
-
- Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. . International council for standardization in haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–50. - PubMed
-
- Mavrakanas T, Bounameaux H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol Ther. 2011;130(1):46–58. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

