Healthy grocery delivery in the usual care for adults recovering from an acute coronary event: protocol for a three-arm randomised controlled trial
- PMID: 38035748
- PMCID: PMC10689354
- DOI: 10.1136/bmjopen-2023-074278
Healthy grocery delivery in the usual care for adults recovering from an acute coronary event: protocol for a three-arm randomised controlled trial
Abstract
Introduction: Coronary heart disease is a major contributor to the global burden of disease. Appropriate nutrition is a cornerstone of the prevention and treatment of coronary heart disease; however, barriers including cost and access to recommended foods limits long-term adherence for many. We are conducting, in adults with coronary heart disease, a randomised controlled trial comparing usual care with two dietary interventions in which usual care is augmented by 12 weeks free delivered groceries.
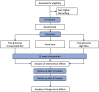
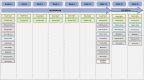
Methods and analysis: Three hundred adults recovering from an acute coronary event will be recruited from outpatient cardiovascular services in three regions of Aotearoa New Zealand. Participants will be randomly allocated to three arms: usual care (control group), usual care and the free delivery of foods high in dietary fibre or usual care and the free delivery of foods high in unsaturated fats. Interventions duration is 12 weeks, with a further 12 months follow-up. The primary outcome measures are change in low-density lipoprotein (LDL) cholesterol concentration following the intervention, and a cost-effectiveness analysis of healthcare access and social costs in the year after the intervention. A broad range of secondary outcome measures include other blood lipids, anthropometry, glycaemia, inflammatory markers, gut microbiome, dietary biomarkers, food acceptability, dietary change and the facilitators and barriers to dietary change. The trial will determine whether the free provision of groceries known to reduce cardiovascular risk within usual care will be clinically beneficial and justify the cost of doing so. Results may also provide an indication of the relative benefit of foods rich in dietary fibre or unsaturated fats in coronary heart disease management.
Ethics and dissemination: This trial, The Healthy Heart Study, has Health and Disability Ethics Committee approval (20/NTB/121), underwent Māori consultation, and has locality authority to be conducted in Canterbury, Otago and Southland.
Trial registration number: ACTRN12620000689976, U1111-1250-1499.
Keywords: Coronary heart disease; NUTRITION & DIETETICS; PUBLIC HEALTH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1659–724. 10.1016/S0140-6736(16)31679-8 - DOI - PMC - PubMed
-
- Kassebaum NJ, Arora M, Barber RM, et al. Global, regional, and national disability-adjusted life-years (Dalys) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1603–58. 10.1016/S0140-6736(16)31460-X - DOI - PMC - PubMed
-
- World Health Organization . Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2020. World Health Organization, 2013.
-
- Mann J, Truswell S, Hodson L. Essentials of Human Nutrition 6e. Oxford, UK: Oxford University Press, 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources