Bile acid metabolomics identifies chenodeoxycholic acid as a therapeutic agent for pancreatic necrosis
- PMID: 38035885
- PMCID: PMC10772342
- DOI: 10.1016/j.xcrm.2023.101304
Bile acid metabolomics identifies chenodeoxycholic acid as a therapeutic agent for pancreatic necrosis
Abstract
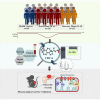
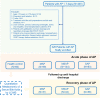
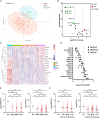
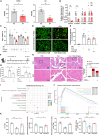
Bile acids are altered and associated with prognosis in patients with acute pancreatitis (AP). Here, we conduct targeted metabolomic analyses to detect bile acids changes in patients during the acute (n = 326) and the recovery (n = 133) phases of AP, as well as in healthy controls (n = 60). Chenodeoxycholic acid (CDCA) decreases in the acute phase, increases in the recovery phase, and is associated with pancreatic necrosis. CDCA and its derivative obeticholic acid exhibit a protective effect against acinar cell injury in vitro and pancreatic necrosis in murine models, and RNA sequencing reveals that the oxidative phosphorylation pathway is mainly involved. Moreover, we find that overexpression of farnesoid X receptor (FXR, CDCA receptor) inhibits pancreatic necrosis, and interfering expression of FXR exhibits an opposite phenotype in mice. Our results possibly suggest that targeting CDCA is a potential strategy for the treatment of acinar cell necrosis in AP, but further verification is needed.
Keywords: acinar cells; acute pancreatitis; bile acids metabolomics; chenodeoxycholic acid; farnesoid X receptor; obeticholic acid; oxidative phosphorylation; pancreatic necrosis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Mederos M.A., Reber H.A., Girgis M.D. Acute Pancreatitis: A Review. JAMA. 2021;325:382–390. - PubMed
-
- Lee P.J., Papachristou G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:479–496. - PubMed
-
- Yasuda I., Takahashi K. Endoscopic management of walled-off pancreatic necrosis. Dig. Endosc. 2021;33:335–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

