Fully magnetically centrifugal left ventricular assist device and long-term outcomes: the ELEVATE registry
- PMID: 38036414
- PMCID: PMC10959573
- DOI: 10.1093/eurheartj/ehad658
Fully magnetically centrifugal left ventricular assist device and long-term outcomes: the ELEVATE registry
Abstract
Background and aims: HeartMate 3 (HM3) is a fully magnetically levitated continuous flow left ventricular assist device, which received CE marking in 2015. The ELEVATE Registry was initiated to collect real-world outcomes in patients treated with HM3 post-CE Mark approval.
Methods: A total of 540 subjects implanted at 26 centres between March 2015 and February 2017 were included in this registry. Of these, 463 received the device as a primary implant (primary implant cohort, PIC), 19 as a pump exchange (pump exchange cohort), and in 58 patients, only anonymized survival data were collected (anonymized cohort, AC). Patients in the PIC contributed to the baseline demographics, survival, adverse events, quality of life (QoL) (EuroQoL-5 Dimensions-5 Levels visual analogue scale), and functional capacity (6 min walk distance) assessments, while patients in the AC contributed only to survival.
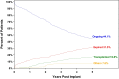
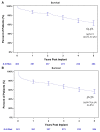
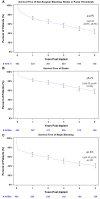
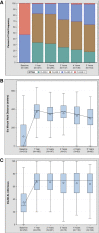
Results: Primary implant cohort patients had a mean age of 56 years and were predominantly male (89%) with 48% ischaemic aetiology. The majority of subjects was designated bridge to transplant (66%) and had INTERMACS Profiles 1-3 (70%). At baseline, the subjects had poor functional capacity (104 ± 140 m) and impaired QoL (35 ± 19 points). The overall survival rate of the PIC was 63.3% and survival free of stroke was 58.1% at 5 years. Significant improvements in functional capacity and QoL were observed and maintained for 5 years (301 ± 131 m and 64 ± 20 points, respectively).
Conclusions: Real-world data from the ELEVATE registry demonstrate an overall survival rate for primary implants of 63.3%. In the PIC, reductions in adverse events for patients in the extended follow-up and improved QoL and functional capacity were observed at 5 years in this patient population with advanced heart failure.
Keywords: Cardiac surgery; Chronic heart failure; HF surgery; Heart failure; HeartMate 3; Left ventricular assist device; Mechanical circulatory support.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
The transformative potential of left ventricular assist devices in advanced heart failure: no more a therapeutic orphan.Eur Heart J. 2024 Feb 21;45(8):626-628. doi: 10.1093/eurheartj/ehad555. Eur Heart J. 2024. PMID: 38073194 Free PMC article. No abstract available.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

