Treatment for First Cytomegalovirus Infection Post-Hematopoietic Cell Transplant in the AURORA Trial: A Multicenter, Double-Blind, Randomized, Phase 3 Trial Comparing Maribavir With Valganciclovir
- PMID: 38036487
- PMCID: PMC10954327
- DOI: 10.1093/cid/ciad709
Treatment for First Cytomegalovirus Infection Post-Hematopoietic Cell Transplant in the AURORA Trial: A Multicenter, Double-Blind, Randomized, Phase 3 Trial Comparing Maribavir With Valganciclovir
Erratum in
-
Correction to: Treatment for First Cytomegalovirus Infection Post-Hematopoietic Cell Transplant in the AURORA Trial: A Multicenter, Double-Blind, Randomized, Phase 3 Trial Comparing Maribavir With Valganciclovir.Clin Infect Dis. 2024 Sep 26;79(3):803. doi: 10.1093/cid/ciae356. Clin Infect Dis. 2024. PMID: 39095060 Free PMC article. No abstract available.
Abstract
Background: Neutropenia may limit the use of valganciclovir treatment for cytomegalovirus (CMV) infection following hematopoietic cell transplant (HCT). A phase 2 study indicated efficacy of maribavir with fewer treatment-limiting toxicities than valganciclovir.
Methods: In this multicenter, double-blind, phase 3 study, patients with first asymptomatic CMV infection post-HCT were stratified and randomized 1:1 to maribavir 400 mg twice daily or valganciclovir (dose-adjusted for renal clearance) for 8 weeks with 12 weeks of follow-up. The primary endpoint was confirmed CMV viremia clearance at week 8 (primary hypothesis of noninferiority margin of 7.0%). The key secondary endpoint was a composite of the primary endpoint with no findings of CMV tissue-invasive disease at week 8 through week 16. Treatment-emergent adverse events (TEAEs) were assessed.
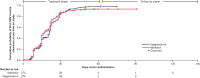
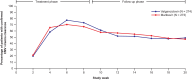
Results: Among patients treated (273 maribavir; 274 valganciclovir), the primary endpoint of noninferiority of maribavir was not met (maribavir, 69.6%; valganciclovir, 77.4%; adjusted difference: -7.7%; 95% confidence interval [CI]: -14.98, -.36; lower limit of 95% CI of treatment difference exceeded -7.0%). At week 16, 52.7% and 48.5% of patients treated (maribavir and valganciclovir, respectively) maintained CMV viremia clearance without tissue-invasive disease (adjusted difference: 4.4%; 95% CI: -3.91, 12.76). With maribavir (vs valganciclovir), fewer patients experienced neutropenia (16.1% and 52.9%) or discontinued due to TEAEs (27.8% and 41.2%). Discontinuations were mostly due to neutropenia (maribavir, 4.0%; valganciclovir, 17.5%).
Conclusions: Although noninferiority of maribavir to valganciclovir for the primary endpoint was not achieved based on the prespecified noninferiority margin, maribavir demonstrated comparable CMV viremia clearance during post-treatment follow-up, with fewer discontinuations due to neutropenia. Clinical Trials Registration. NCT02927067 [AURORA].
Keywords: cytomegalovirus; hematopoietic cell transplant; maribavir; valganciclovir.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. G. A. P.—research funding (paid to their institution): Merck, Takeda; consulting fees: Merck, Symbio, Takeda; honoraria: Merck; data safety monitoring board or advisory board: AlloVir, Armata, Vera; chair (voluntary): Transplant Infectious Disease of the American Society for Transplantation and Cellular Therapy (ASTCT). R. K. A.—study/grant support (paid to their institution): AiCuris, Astellas, AstraZeneca, Chimerix, Merck, Oxford Immunotec, Qiagen, Regeneron, Takeda. C. C.—study/grant support (paid to their institution): Merck Sharp & Dohme, Takeda; consulting fees: Takeda; honoraria (personal): Takeda, Merck Sharp & Dohme. R. F. D.—consulting fees (advisor honoraria): Cidara, Gilead Sciences, Jazz Pharmaceuticals, Merck Sharp & Dohme, Omeros Corporation, Sobi; honoraria (speaker’s bureau): Bristol Myers Squibb, Gilead Sciences, Jazz Pharmaceuticals, Merck Sharp & Dohme, Omeros Corporation, Takeda; support for attending meetings and/or travel: Bristol Myers Squibb, Gilead Sciences, Jazz Pharmaceuticals, Kite Pharma, Merck Sharp & Dohme, Omeros Corporation, Pfizer; receipt of equipment and materials: Roche Diagnostics. S. H.—honoraria (advisory board): Takeda. J. M.—honoraria (speaker) and advisory board participation: Takeda; consulting fees: F2G, Gilead Sciences, Mundipharma, Pfizer; honoraria: F2G, Gilead Sciences, Mundipharma, Pfizer; support for attending meetings and/or travel: F2G, Gilead Sciences, Mundipharma; data safety monitoring board or advisory board: Cidara, F2G, Gilead Sciences, Mundipharma, Takeda. C. S.—honoraria (speaker): GSK, Merck Sharp & Dohme, Pfizer; support for attending meetings and/or travel to American Society of Hematology Annual Meeting: Kite Pharma, a Gilead company. J.-A. H. Y.—trial reimbursement (paid to their institution): Takeda; Editor in Chief (completed 30 June 2022): Clinical Microbiology Reviews; Associate Editor (unpaid): Transplantation and Cellular Therapy. M. F., J. W.—employee and stock/stock options: Takeda. R. A. M.—employee: Takeda. D. J. W.—research grants: Ansun Biopharma, Cidara, Symbio, Takeda; consulting fees: Shire, ViroPharma. K. S. P. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures




References
-
- Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019; 19:e260–72. - PubMed
-
- Peffault De Latour R, Chevallier P, Blaise D, et al. Clinical and economic impact of treated CMV infection in adult CMV-seropositive patients after allogeneic hematopoietic cell transplantation. J Med Virol 2020; 92:3665–73. - PubMed
-
- Ota R, Hirata A. Relationship between renal dysfunction and electrolyte abnormalities in hematopoietic stem cell transplant patients treated with foscarnet. J Chemother 2021; 33:539–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

