A dynamic partitioning mechanism polarizes membrane protein distribution
- PMID: 38036511
- PMCID: PMC10689845
- DOI: 10.1038/s41467-023-43615-2
A dynamic partitioning mechanism polarizes membrane protein distribution
Abstract
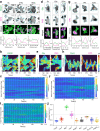
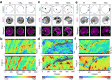
The plasma membrane is widely regarded as the hub of the numerous signal transduction activities. Yet, the fundamental biophysical mechanisms that spatiotemporally compartmentalize different classes of membrane proteins remain unclear. Using multimodal live-cell imaging, here we first show that several lipid-anchored membrane proteins are consistently depleted from the membrane regions where the Ras/PI3K/Akt/F-actin network is activated. The dynamic polarization of these proteins does not depend upon the F-actin-based cytoskeletal structures, recurring shuttling between membrane and cytosol, or directed vesicular trafficking. Photoconversion microscopy and single-molecule measurements demonstrate that these lipid-anchored molecules have substantially dissimilar diffusion profiles in different regions of the membrane which enable their selective segregation. When these diffusion coefficients are incorporated into an excitable network-based stochastic reaction-diffusion model, simulations reveal that the altered affinity mediated selective partitioning is sufficient to drive familiar propagating wave patterns. Furthermore, normally uniform integral and lipid-anchored membrane proteins partition successfully when membrane domain-specific peptides are optogenetically recruited to them. We propose "dynamic partitioning" as a new mechanism that can account for large-scale compartmentalization of a wide array of lipid-anchored and integral membrane proteins during various physiological processes where membrane polarizes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Update of
-
A dynamic partitioning mechanism polarizes membrane protein distribution.bioRxiv [Preprint]. 2023 Jan 3:2023.01.03.522496. doi: 10.1101/2023.01.03.522496. bioRxiv. 2023. Update in: Nat Commun. 2023 Nov 30;14(1):7909. doi: 10.1038/s41467-023-43615-2. PMID: 36712016 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

