Harnessing synthetic biology for advancing RNA therapeutics and vaccine design
- PMID: 38036580
- PMCID: PMC10689799
- DOI: 10.1038/s41540-023-00323-3
Harnessing synthetic biology for advancing RNA therapeutics and vaccine design
Abstract
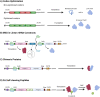
Recent global events have drawn into focus the diversity of options for combatting disease across a spectrum of prophylactic and therapeutic approaches. The recent success of the mRNA-based COVID-19 vaccines has paved the way for RNA-based treatments to revolutionize the pharmaceutical industry. However, historical treatment options are continuously updated and reimagined in the context of novel technical developments, such as those facilitated through the application of synthetic biology. When it comes to the development of genetic forms of therapies and vaccines, synthetic biology offers diverse tools and approaches to influence the content, dosage, and breadth of treatment with the prospect of economic advantage provided in time and cost benefits. This can be achieved by utilizing the broad tools within this discipline to enhance the functionality and efficacy of pharmaceutical agent sequences. This review will describe how synthetic biology principles can augment RNA-based treatments through optimizing not only the vaccine antigen, therapeutic construct, therapeutic activity, and delivery vector. The enhancement of RNA vaccine technology through implementing synthetic biology has the potential to shape the next generation of vaccines and therapeutics.
© 2023. The Author(s).
Conflict of interest statement
C.H.J. reports that they are an employee of Pfizer Inc. and may hold stock or stock options in the company. C.H.J. reports that they are an employee of Pfizer Inc. and may hold stock or stock options in the company. M.B. and A.B.H. reports that they received financial compensation for consultancy and technical writing services.
Figures


References
-
- Erlich, H. A. PCR Technology - Principles and Applications for DNA Amplification. Vol. 246 (Palgrave Macmillan London, 1989).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

