Low-dose brain radiation: lowering hyperphosphorylated-tau without increasing DNA damage or oncogenic activation
- PMID: 38036591
- PMCID: PMC10689500
- DOI: 10.1038/s41598-023-48146-w
Low-dose brain radiation: lowering hyperphosphorylated-tau without increasing DNA damage or oncogenic activation
Abstract
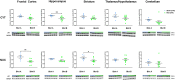
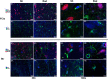
Brain radiation has been medically used to alter the metabolism of cancerous cells and induce their elimination. Rarely, though, brain radiation has been used to interfere with the pathomechanisms of non-cancerous brain disorders, especially neurodegenerative disorders. Data from low-dose radiation (LDR) on swine brains demonstrated reduced levels of phosphorylated-tau (CP13) and amyloid precursor protein (APP) in radiated (RAD) versus sham (SH) animals. Phosphorylated-tau and APP are involved in Alzheimer's disease (AD) pathogenesis. We determined if the expression levels of hyperphosphorylated-tau, 3R-tau, 4R-tau, synaptic, intraneuronal damage, and DNA damage/oncogenic activation markers were altered in RAD versus SH swine brains. Quantitative analyses demonstrated reduced levels of AT8 and 3R-tau in hippocampus (H) and striatum (Str), increased levels of synaptophysin and PSD-95 in frontal cortex (FCtx), and reduced levels of NF-L in cerebellum (CRB) of RAD versus SH swine. DNA damage and oncogene activation markers levels did not differ between RAD and SH animals, except for histone-H3 (increased in FCtx and CRB, decreased in Str), and p53 (reduced in FCtx, Str, H and CRB). These findings confirm the region-based effects of sLDR on proteins normally expressed in larger mammalian brains and support the potential applicability of LDR to beneficially interfere against neurodegenerative mechanisms.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

