The evolution of short- and long-range weapons for bacterial competition
- PMID: 38036633
- PMCID: PMC10697841
- DOI: 10.1038/s41559-023-02234-2
The evolution of short- and long-range weapons for bacterial competition
Abstract
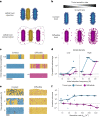
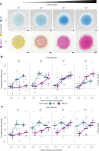
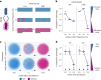
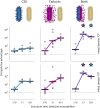
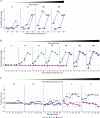
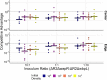
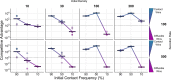
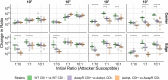
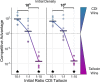
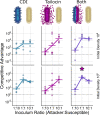
Bacteria possess a diverse range of mechanisms for inhibiting competitors, including bacteriocins, tailocins, type VI secretion systems and contact-dependent inhibition (CDI). Why bacteria have evolved such a wide array of weapon systems remains a mystery. Here we develop an agent-based model to compare short-range weapons that require cell-cell contact, with long-range weapons that rely on diffusion. Our model predicts that contact weapons are useful when an attacking strain is outnumbered, facilitating invasion and establishment. By contrast, ranged weapons tend to be effective only when attackers are abundant. We test our predictions with the opportunistic pathogen Pseudomonas aeruginosa, which naturally carries multiple weapons, including CDI and diffusing tailocins. As predicted, short-range CDI can function at low and high frequencies, while long-range tailocins require high frequency and cell density to function effectively. Head-to-head competition experiments with the two weapon types further support our predictions: a tailocin attacker defeats CDI only when it is numerically dominant, but then we find it can be devastating. Finally, we show that the two weapons work well together when one strain employs both. We conclude that short- and long-range weapons serve different functions and allow bacteria to fight both as individuals and as a group.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Emlen, D. Animal Weapons: The Evolution of Battle (Picador, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

