Rapid fluctuations in functional connectivity of cortical networks encode spontaneous behavior
- PMID: 38036743
- PMCID: PMC11316935
- DOI: 10.1038/s41593-023-01498-y
Rapid fluctuations in functional connectivity of cortical networks encode spontaneous behavior
Abstract
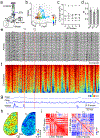
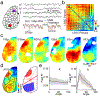
Experimental work across species has demonstrated that spontaneously generated behaviors are robustly coupled to variations in neural activity within the cerebral cortex. Functional magnetic resonance imaging data suggest that temporal correlations in cortical networks vary across distinct behavioral states, providing for the dynamic reorganization of patterned activity. However, these data generally lack the temporal resolution to establish links between cortical signals and the continuously varying fluctuations in spontaneous behavior observed in awake animals. Here, we used wide-field mesoscopic calcium imaging to monitor cortical dynamics in awake mice and developed an approach to quantify rapidly time-varying functional connectivity. We show that spontaneous behaviors are represented by fast changes in both the magnitude and correlational structure of cortical network activity. Combining mesoscopic imaging with simultaneous cellular-resolution two-photon microscopy demonstrated that correlations among neighboring neurons and between local and large-scale networks also encode behavior. Finally, the dynamic functional connectivity of mesoscale signals revealed subnetworks not predicted by traditional anatomical atlas-based parcellation of the cortex. These results provide new insights into how behavioral information is represented across the neocortex and demonstrate an analytical framework for investigating time-varying functional connectivity in neural networks.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests Statement
The authors declare no conflicts of interest exist.
Figures



References
-
- Breakspear M Dynamic models of large-scale brain activity. Nat Neurosci 20, 340–352 (2017). - PubMed

