BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance
- PMID: 38036767
- PMCID: PMC10932731
- DOI: 10.1038/s41590-023-01700-0
BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance
Abstract
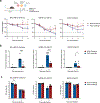
Bacille Calmette-Guérin (BCG) vaccination can confer nonspecific protection against heterologous pathogens. However, the underlying mechanisms remain mysterious. We show that mice vaccinated intravenously with BCG exhibited reduced weight loss and/or improved viral clearance when challenged with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 B.1.351) or PR8 influenza. Protection was first evident between 14 and 21 d post-vaccination and lasted ∼3 months. Notably, BCG induced a biphasic innate response and robust antigen-specific type 1 helper T cell (TH1 cell) responses in the lungs. MyD88 signaling was essential for innate and TH1 cell responses, and protection against SARS-CoV-2. Depletion of CD4+ T cells or interferon (IFN)-γ activity before infection obliterated innate activation and protection. Single-cell and spatial transcriptomics revealed CD4-dependent expression of IFN-stimulated genes in lung myeloid and epithelial cells. Notably, BCG also induced protection against weight loss after mouse-adapted SARS-CoV-2 BA.5, SARS-CoV and SHC014 coronavirus infections. Thus, BCG elicits integrated organ immunity, where CD4+ T cells feed back on tissue myeloid and epithelial cells to imprint prolonged and broad innate antiviral resistance.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS
B.P. has served or is serving on the External Immunology Network of GSK, and on the scientific advisory boards of Sanofi, Medicago, CircBio and Boehringer-Ingelheim. R.S.B. serves on the Scientific Advisory Board of Takeda, VaxArt, and Invivyd and has collaborations with Janssen Pharmaceuticals, Gilead, Chimerix, and Pardes Biosciences. S.R.L. and R.S.B. are listed on a patent for the SARS-CoV-2 MA10 virus (US 11225508 B1, “Mouse-adapted SARS-CoV-2 Viruses and Methods of Use Thereof”). G.P.N is a co-founder and stockholder of Ionpath Inc.; G.P.N. is a co-founder and stockholder of Akoya Biosciences, Inc. and an inventor on patent US9909167.; G.P.N. is a Scientific Advisory Board member for Akoya Biosciences, Inc.; G.P.N. received research grants from Pfizer, Inc.; Vaxart, Inc.; Celgene, Inc.; and Juno Therapeutics, Inc. G.P.N is co-founder for Scale Biosciences Inc. M.S.S. has served in an advisory role for Ocugen, Inc. The remaining authors declare no competing interests.
Figures




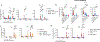


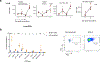


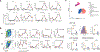



References
-
- Aaby P et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis 204, 245–252 (2011). - PubMed
-
- Moorlag SJCFM, Arts RJW, van Crevel R & Netea MG Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect 25, 1473–1478 (2019). - PubMed
METHODS-ONLY REFERENCES
-
- Szretter KJ, Balish AL & Katz JM Influenza: Propagation, Quantification, and Storage. Curr. Protoc. Microbiol 3, (2006). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

