GNF-7, a novel FLT3 inhibitor, overcomes drug resistance for the treatment of FLT3‑ITD acute myeloid leukemia
- PMID: 38037057
- PMCID: PMC10691066
- DOI: 10.1186/s12935-023-03142-y
GNF-7, a novel FLT3 inhibitor, overcomes drug resistance for the treatment of FLT3‑ITD acute myeloid leukemia
Abstract
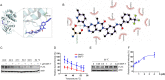
Background: Acute myeloid leukemia (AML) with FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation accounts for a large proportion of AML patients and diagnosed with poor prognosis. Although the prognosis of FLT3-ITD AML has been greatly improved, the drug resistance frequently occurred in the treatment of FLT3 targeting drugs. GNF-7, a multitargeted kinase inhibitor, which provided a novel therapeutic strategy for overriding leukemia. In this study, we explored the antitumor activity of GNF-7 against FLT3-ITD and clinically-relevant drug resistance in FLT3 mutant AML.
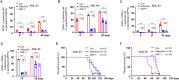
Methods: Growth inhibitory assays were performed in AML cell lines and Ba/F3 cells expressing various FLT3 mutants to evaluate the antitumor activity of GNF-7 in vitro. Western blotting was used to examine the inhibitory effect of GNF-7 on FLT3 and its downstream pathways. Molecular docking and cellular thermal shift assay (CETSA) were performed to demonstrate the binding of FLT3 to GNF-7. The survival benefit of GNF-7 in vivo was assessed in mouse models of transformed Ba/F3 cells harboring FLT3-ITD and FLT3-ITD/F691L mutation. Primary patient samples and a patient-derived xenograft (PDX) model were also used to determine the efficacy of GNF-7.
Results: GNF-7 inhibited the cell proliferation of Ba/F3 cells expressing FLT3-ITD and exhibited potently anti-leukemia activity on primary FLT3-ITD AML samples. Moreover, GNF-7 could bind to FLT3 protein and inhibit the downstream signaling pathway activated by FLT3 including STAT5, PI3K/AKT and MAPK/ERK. In vitro and in vivo studies showed that GNF-7 exhibited potent inhibitory activity against FLT3-ITD/F691L that confers resistant to quizartinib (AC220) or gilteritinib. Importantly, GNF-7 showed potent cytotoxic effect on leukemic stem cells, significantly extend the survival of PDX model and exhibited similar therapy effect compared with gilteritinib.
Conclusions: Our results show that GNF-7 is a potent FLT3-ITD inhibitor and may become a promising lead compound applied for treating some of the clinically drug resistant patients.
Keywords: Acute myeloid leukemia; Drug resistance; FLT3-ITD; GNF-7.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ningetinib, a novel FLT3 inhibitor, overcomes secondary drug resistance in acute myeloid leukemia.Cell Commun Signal. 2024 Jul 8;22(1):355. doi: 10.1186/s12964-024-01729-0. Cell Commun Signal. 2024. PMID: 38978049 Free PMC article.
-
Sitravatinib as a potent FLT3 inhibitor can overcome gilteritinib resistance in acute myeloid leukemia.Biomark Res. 2023 Jan 24;11(1):8. doi: 10.1186/s40364-022-00447-4. Biomark Res. 2023. PMID: 36691065 Free PMC article.
-
Heat shock protein 90 inhibitors overcome the resistance to Fms-like tyrosine kinase 3 inhibitors in acute myeloid leukemia.Oncotarget. 2018 Sep 28;9(76):34240-34258. doi: 10.18632/oncotarget.26045. eCollection 2018 Sep 28. Oncotarget. 2018. PMID: 30344940 Free PMC article.
-
Quizartinib (AC220): a promising option for acute myeloid leukemia.Drug Des Devel Ther. 2019 Apr 8;13:1117-1125. doi: 10.2147/DDDT.S198950. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31114157 Free PMC article. Review.
-
Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia.Biomark Res. 2019 Sep 11;7:19. doi: 10.1186/s40364-019-0170-2. eCollection 2019. Biomark Res. 2019. PMID: 31528345 Free PMC article. Review.
Cited by
-
USP24 promotes hepatocellular carcinoma progression by deubiquitinating and stabilizing YAP1.Cancer Cell Int. 2025 Apr 26;25(1):164. doi: 10.1186/s12935-025-03796-w. Cancer Cell Int. 2025. PMID: 40287768 Free PMC article.
-
Long noncoding RNAs in acute myeloid leukemia: biomarkers, prognostic indicators, and treatment potential.Cancer Cell Int. 2025 Apr 5;25(1):131. doi: 10.1186/s12935-025-03763-5. Cancer Cell Int. 2025. PMID: 40188050 Free PMC article. Review.
References
-
- Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735–741. doi: 10.1200/JCO.2011.36.9868. - DOI - PubMed
-
- Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

