Neoantigen-targeted TCR-engineered T cell immunotherapy: current advances and challenges
- PMID: 38037114
- PMCID: PMC10690996
- DOI: 10.1186/s40364-023-00534-0
Neoantigen-targeted TCR-engineered T cell immunotherapy: current advances and challenges
Abstract
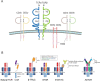
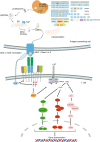
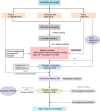
Adoptive cell therapy using T cell receptor-engineered T cells (TCR-T) is a promising approach for cancer therapy with an expectation of no significant side effects. In the human body, mature T cells are armed with an incredible diversity of T cell receptors (TCRs) that theoretically react to the variety of random mutations generated by tumor cells. The outcomes, however, of current clinical trials using TCR-T cell therapies are not very successful especially involving solid tumors. The therapy still faces numerous challenges in the efficient screening of tumor-specific antigens and their cognate TCRs. In this review, we first introduce TCR structure-based antigen recognition and signaling, then describe recent advances in neoantigens and their specific TCR screening technologies, and finally summarize ongoing clinical trials of TCR-T therapies against neoantigens. More importantly, we also present the current challenges of TCR-T cell-based immunotherapies, e.g., the safety of viral vectors, the mismatch of T cell receptor, the impediment of suppressive tumor microenvironment. Finally, we highlight new insights and directions for personalized TCR-T therapy.
Keywords: Neoantigen; Neoantigen-reactive TCRs; TCR-T.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neoantigen-targeted TCR-T cell therapy for solid tumors: How far from clinical application.Cancer Lett. 2022 Oct 10;546:215840. doi: 10.1016/j.canlet.2022.215840. Epub 2022 Jul 31. Cancer Lett. 2022. PMID: 35921969 Review.
-
Induction of Neoantigen-Specific Cytotoxic T Cells and Construction of T-cell Receptor-Engineered T Cells for Ovarian Cancer.Clin Cancer Res. 2018 Nov 1;24(21):5357-5367. doi: 10.1158/1078-0432.CCR-18-0142. Epub 2018 May 2. Clin Cancer Res. 2018. PMID: 29720506
-
Generation of neoantigen-specific T cells for adoptive cell transfer for treating head and neck squamous cell carcinoma.Oncoimmunology. 2021 May 25;10(1):1929726. doi: 10.1080/2162402X.2021.1929726. Oncoimmunology. 2021. PMID: 34104546 Free PMC article.
-
Neoantigen-specific TCR-T cell-based immunotherapy for acute myeloid leukemia.Exp Hematol Oncol. 2022 Nov 16;11(1):100. doi: 10.1186/s40164-022-00353-3. Exp Hematol Oncol. 2022. PMID: 36384590 Free PMC article. Review.
-
Evolution of CD8+ T Cell Receptor (TCR) Engineered Therapies for the Treatment of Cancer.Cells. 2021 Sep 10;10(9):2379. doi: 10.3390/cells10092379. Cells. 2021. PMID: 34572028 Free PMC article. Review.
Cited by
-
Ezrin's role in gastric cancer progression: Implications for immune microenvironment modulation and therapeutic potential.Heliyon. 2024 Feb 28;10(5):e27155. doi: 10.1016/j.heliyon.2024.e27155. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38449647 Free PMC article.
-
Personalized nanovaccines for treating solid cancer metastases.J Hematol Oncol. 2024 Nov 28;17(1):115. doi: 10.1186/s13045-024-01628-4. J Hematol Oncol. 2024. PMID: 39609851 Free PMC article. Review.
-
Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells.Cancers (Basel). 2024 Jul 7;16(13):2478. doi: 10.3390/cancers16132478. Cancers (Basel). 2024. PMID: 39001539 Free PMC article.
-
Novel immunotherapeutic approaches in gastric cancer.Precis Clin Med. 2024 Sep 19;7(4):pbae020. doi: 10.1093/pcmedi/pbae020. eCollection 2024 Dec. Precis Clin Med. 2024. PMID: 39397869 Free PMC article. Review.
-
Personalized, autologous neoantigen-specific T cell therapy in metastatic melanoma: a phase 1 trial.Nat Med. 2025 Mar;31(3):881-893. doi: 10.1038/s41591-024-03418-4. Epub 2025 Jan 3. Nat Med. 2025. PMID: 39753970 Free PMC article. Clinical Trial.
References
-
- Liang W, Yi R, Wang W, Shi Y, Zhang J, Xu X, et al. Enhancing the Antitumor Immunity of T Cells by Engineering the Lipid-Regulatory Site of the TCR/CD3 Complex. Cancer Immunol Res. 2023;11(1). - PubMed
Publication types
Grants and funding
- 82303544/National Natural Science Foundation of China
- 31870829/National Natural Science Foundation of China
- 82073216/National Natural Science Foundation of China
- 20S11906300/Projection of Shanghai Science and Technology Committee
- 2019CXJQ02/Shanghai Municipal Health Commission, Collaborative Innovation Cluster Project
LinkOut - more resources
Full Text Sources

