Improving the Conductivity of Amide-Based Small Molecules through Enhanced Molecular Packing and Their Application as Hole Transport Mediators in Perovskite Solar Cells
- PMID: 38037633
- PMCID: PMC10685326
- DOI: 10.1021/acsaem.3c01988
Improving the Conductivity of Amide-Based Small Molecules through Enhanced Molecular Packing and Their Application as Hole Transport Mediators in Perovskite Solar Cells
Abstract
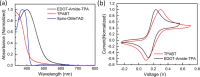
Organic-inorganic hybrid halide perovskite solar cells (PSCs) have attracted substantial attention from the photovoltaic research community, with the power conversion efficiency (PCE) already exceeding 26%. Current state-of-the-art devices rely on Spiro-OMeTAD as the hole-transporting material (HTM); however, Spiro-OMeTAD is costly due to its complicated synthesis and expensive product purification, while its low conductivity ultimately limits the achievable device efficiency. In this work, we build upon our recently introduced family of low-cost amide-based small molecules and introduce a molecule (termed TPABT) that results in high conductivity values (∼10-5 S cm-1 upon addition of standard ionic additives), outperforming our previous amide-based material (EDOT-Amide-TPA, ∼10-6 S cm-1) while only costing an estimated $5/g. We ascribe the increased optoelectronic properties to favorable molecular packing, as shown by single-crystal X-ray diffraction, which results in close spacing between the triphenylamine blocks. This, in turn, results in a short hole-hopping distance between molecules and therefore good mobility and conductivity. In addition, TPABT exhibits a higher bandgap and is as a result more transparent in the visible range of the solar spectrum, leading to lower parasitic absorption losses than Spiro-OMeTAD, and has increased moisture stability. We applied the molecule in perovskite solar cells and obtained good efficiency values in the ∼15% range. Our approach shows that engineering better molecular packing may be the key to developing high-efficiency, low-cost HTMs for perovskite solar cells.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Yin X.; Song Z.; Li Z.; Tang W. Toward Ideal Hole Transport Materials: A Review on Recent Progress in Dopant-Free Hole Transport Materials for Fabricating Efficient and Stable Perovskite Solar Cells. Energy Environ. Sci. 2020, 13 (11), 4057–4086. 10.1039/D0EE02337J. - DOI
-
- NREL Best Research-Cell Efficiency Chart. 2022.
-
- Li S.; Cao Y. L.; Li W. H.; Bo Z. S. A Brief Review of Hole Transporting Materials Commonly Used in Perovskite Solar Cells. Rare Met. 2021, 40 (10), 2712–2729. 10.1007/s12598-020-01691-z. - DOI
LinkOut - more resources
Full Text Sources
