Membrane transporters in cell physiology, cancer metabolism and drug response
- PMID: 38037877
- PMCID: PMC10695176
- DOI: 10.1242/dmm.050404
Membrane transporters in cell physiology, cancer metabolism and drug response
Abstract
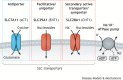
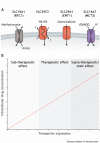
By controlling the passage of small molecules across lipid bilayers, membrane transporters influence not only the uptake and efflux of nutrients, but also the metabolic state of the cell. With more than 450 members, the Solute Carriers (SLCs) are the largest transporter super-family, clustering into families with different substrate specificities and regulatory properties. Cells of different types are, therefore, able to tailor their transporter expression signatures depending on their metabolic requirements, and the physiological importance of these proteins is illustrated by their mis-regulation in a number of disease states. In cancer, transporter expression is heterogeneous, and the SLC family has been shown to facilitate the accumulation of biomass, influence redox homeostasis, and also mediate metabolic crosstalk with other cell types within the tumour microenvironment. This Review explores the roles of membrane transporters in physiological and malignant settings, and how these roles can affect drug response, through either indirect modulation of sensitivity or the direct transport of small-molecule therapeutic compounds into cells.
Keywords: Cancer Metabolism; Drug Uptake; Pharmacology; Transporters.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

