The immunogenicity and the safety of the adjuvanted glycoprotein E (gE)-based recombinant vaccine against herpes zoster (RZV) in cancer patients during immunotherapy
- PMID: 38037900
- PMCID: PMC10732600
- DOI: 10.1080/21645515.2023.2288282
The immunogenicity and the safety of the adjuvanted glycoprotein E (gE)-based recombinant vaccine against herpes zoster (RZV) in cancer patients during immunotherapy
Abstract
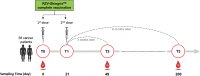
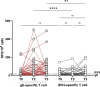
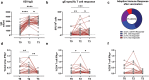
Herpes zoster (HZ) is caused by the reactivation of latent varicella zoster virus (VZV). Severe immunocompromising conditions, such as solid tumors, have been largely associated with an increased risk for HZ due to waning VZV-specific cellular immunity. With the approval of the adjuvanted glycoprotein E (gE)-based recombinant vaccine (RZV; Shingrix™, GSK) also in immunocompromised subjects, HZ is considered a vaccine-preventable disease changing perspectives in immunocompromised subjects. To date, no clinical trial has evaluated the immunogenicity in the patients with cancer undergoing immunotherapy. In this study, we describe the humoral and cell-mediated immune responses in 38 cancer patients treated with immune checkpoint inhibitors (ICIs) and receiving RZV. We used samples collected at baseline (T0), 3 weeks (T2), and 6 months (T3) after the complete RV vaccination schedule. Our data showed that a significant proportion (40,5%) of RZV recipients mounted a stronger humoral and cell-mediated immune response at 3 weeks (T2) after complete RZV vaccination schedule. Interestingly, both humoral and cell-mediated immune responses were mostly stable over 6 months (T3). Interestingly, the overall IFNγ-producing lymphocytes was mainly associated with CD4 T cell response (p = .0012). In conclusion, data from our pilot study suggest a strong and long-lasting immunogenicity of RZV in ICI-treated patients. Prospective analyses at 1 year after vaccination will be performed in order to evaluate the long-term persistence of humoral and cell-mediated response against RZV.
Keywords: CD4; CD8; PHN; RZV; cancer; herpes zoster; immunotherapy; vaccine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
