Activation of the pentose phosphate pathway in macrophages is crucial for granuloma formation in sarcoidosis
- PMID: 38038136
- PMCID: PMC10688990
- DOI: 10.1172/JCI171088
Activation of the pentose phosphate pathway in macrophages is crucial for granuloma formation in sarcoidosis
Abstract
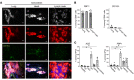
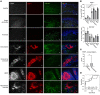
Sarcoidosis is a disease of unknown etiology in which granulomas form throughout the body and is typically treated with glucocorticoids, but there are no approved steroid-sparing alternatives. Here, we investigated the mechanism of granuloma formation using single-cell RNA-Seq in sarcoidosis patients. We observed that the percentages of triggering receptor expressed on myeloid cells 2-positive (TREM2-positive) macrophages expressing angiotensin-converting enzyme (ACE) and lysozyme, diagnostic makers of sarcoidosis, were increased in cutaneous sarcoidosis granulomas. Macrophages in the sarcoidosis lesion were hypermetabolic, especially in the pentose phosphate pathway (PPP). Expression of the PPP enzymes, such as fructose-1,6-bisphosphatase 1 (FBP1), was elevated in both systemic granuloma lesions and serum of sarcoidosis patients. Granuloma formation was attenuated by the PPP inhibitors in in vitro giant cell and in vivo murine granuloma models. These results suggest that the PPP may be a promising target for developing therapeutics for sarcoidosis.
Keywords: Antigen-presenting cells; Dermatology; Immunology; Macrophages; Skin.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

