Early Microbiologic Markers of Pulmonary Tuberculosis Treatment Outcomes
- PMID: 38038600
- PMCID: PMC10704230
- DOI: 10.1513/AnnalsATS.202302-144OC
Early Microbiologic Markers of Pulmonary Tuberculosis Treatment Outcomes
Abstract
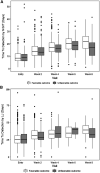
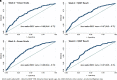
Rationale: Earlier biomarkers of pulmonary tuberculosis (PTB) treatment outcomes are critical to monitor shortened anti-TB treatment (ATT). Objectives: To identify early microbiologic markers of unfavorable TB treatment outcomes. Methods: We performed a subanalysis of 2 prospective TB cohort studies conducted from 2013 to 2019 in India. We included participants aged ⩾18 years who initiated 6-month ATT for clinically or microbiologically diagnosed drug-sensitive PTB and completed at least one follow-up visit. Sputum specimens were subjected to a baseline Xpert Mycobacterium tuberculosis/rifampin (MTB/RIF) assay, acid-fast bacilli (AFB) microscopy and liquid and solid cultures, and serial AFB microscopy and liquid and solid cultures at weeks 2, 4, and 8. Poisson regression was used to assess the impact of available microbiologic markers (test positivity, smear grade, time to detection, and time to conversion) on a composite outcome of failure, recurrence, or death by 18 months after the end of treatment. Models were adjusted for age, sex, nutritional status, diabetes, smoking, alcohol consumption, and regimen type. Results: Among 1,098 eligible cases, there were 251 (22%) adverse TB treatment outcomes: 127 (51%) treatment failures, 73 (29%) recurrences, and 51 (20%) deaths. The primary outcome was independently associated with the Xpert MTB/RIF assay (medium-positive adjusted incidence rate ratio [aIRR], 1.91; 95% confidence interval [CI], 1.07-3.40; high-positive aIRR, 2.51; 95% CI, 1.41-4.46), positive AFB smear (aIRR, 1.48; 95% CI, 1.06-2.06), and positive liquid culture (aIRR, 1.98; 95% CI, 1.21-3.23) at baseline; Week 2 positive liquid culture (aIRR, 1.47; 95% CI, 1.04-2.09); and Week 8 positive AFB smear (aIRR, 1.63; 95% CI, 1.06-2.50) and positive liquid culture (aIRR, 1.54; 95% CI, 1.07-2.22). There was no evidence of Mycobacterium tuberculosis growth in the Mycobacterium Growth Indicator Tube at Week 4 conferring a higher risk of adverse outcomes (aIRR, 1.25; 95% CI, 0.89-1.75). Conclusions: Our analysis identifies Week 2 respiratory mycobacterial culture as the earliest microbiologic marker of unfavorable PTB treatment outcomes.
Keywords: PTB; microbiologic markers; treatment outcomes.
Figures


References
-
- Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe . 2023;4:e20. - PubMed
-
- World Health Organization. Global tuberculosis report 2021. Geneva, Switzerland: World Health Organization; 2021.
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis: rapid diagnostics for tuberculosis detection. Geneva, Switzerland: World Health Organization; 2020. - PubMed
-
- Central TB Division, Directorate General of Health Services. 2017. https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf
-
- Central Tuberculosis Division, Government of India. 2017. https://tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=5507&lid=3528
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

