Cyclophospholipids Enable a Protocellular Life Cycle
- PMID: 38038709
- PMCID: PMC10722605
- DOI: 10.1021/acsnano.3c07706
Cyclophospholipids Enable a Protocellular Life Cycle
Abstract
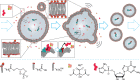
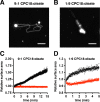
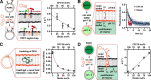
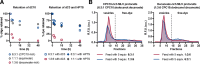
There is currently no plausible path for the emergence of a self-replicating protocell, because prevalent formulations of model protocells are built with fatty acid vesicles that cannot withstand the concentrations of Mg2+ needed for the function and replication of nucleic acids. Although prebiotic chelates increase the survivability of fatty acid vesicles, the resulting model protocells are incapable of growth and division. Here, we show that protocells made of mixtures of cyclophospholipids and fatty acids can grow and divide in the presence of Mg2+-citrate. Importantly, these protocells retain encapsulated nucleic acids during growth and division, can acquire nucleotides from their surroundings, and are compatible with the nonenzymatic extension of an RNA oligonucleotide, chemistry needed for the replication of a primitive genome. Our work shows that prebiotically plausible mixtures of lipids form protocells that are active under the conditions necessary for the emergence of Darwinian evolution.
Keywords: Darwinian evolution; artificial cells; cyclophospholipids; prebiotic chemistry; protocells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

