Deep learning models for automatic tumor segmentation and total tumor volume assessment in patients with colorectal liver metastases
- PMID: 38038829
- PMCID: PMC10692044
- DOI: 10.1186/s41747-023-00383-4
Deep learning models for automatic tumor segmentation and total tumor volume assessment in patients with colorectal liver metastases
Abstract
Background: We developed models for tumor segmentation to automate the assessment of total tumor volume (TTV) in patients with colorectal liver metastases (CRLM).
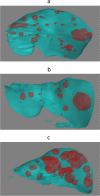
Methods: In this prospective cohort study, pre- and post-systemic treatment computed tomography (CT) scans of 259 patients with initially unresectable CRLM of the CAIRO5 trial (NCT02162563) were included. In total, 595 CT scans comprising 8,959 CRLM were divided into training (73%), validation (6.5%), and test sets (21%). Deep learning models were trained with ground truth segmentations of the liver and CRLM. TTV was calculated based on the CRLM segmentations. An external validation cohort was included, comprising 72 preoperative CT scans of patients with 112 resectable CRLM. Image segmentation evaluation metrics and intraclass correlation coefficient (ICC) were calculated.
Results: In the test set (122 CT scans), the autosegmentation models showed a global Dice similarity coefficient (DSC) of 0.96 (liver) and 0.86 (CRLM). The corresponding median per-case DSC was 0.96 (interquartile range [IQR] 0.95-0.96) and 0.80 (IQR 0.67-0.87). For tumor segmentation, the intersection-over-union, precision, and recall were 0.75, 0.89, and 0.84, respectively. An excellent agreement was observed between the reference and automatically computed TTV for the test set (ICC 0.98) and external validation cohort (ICC 0.98). In the external validation, the global DSC was 0.82 and the median per-case DSC was 0.60 (IQR 0.29-0.76) for tumor segmentation.
Conclusions: Deep learning autosegmentation models were able to segment the liver and CRLM automatically and accurately in patients with initially unresectable CRLM, enabling automatic TTV assessment in such patients.
Relevance statement: Automatic segmentation enables the assessment of total tumor volume in patients with colorectal liver metastases, with a high potential of decreasing radiologist's workload and increasing accuracy and consistency.
Key points: • Tumor response evaluation is time-consuming, manually performed, and ignores total tumor volume. • Automatic models can accurately segment tumors in patients with colorectal liver metastases. • Total tumor volume can be accurately calculated based on automatic segmentations.
Keywords: Artificial intelligence; Colorectal cancer; Deep learning; Liver neoplasms; Tomography (x-ray computed).
© 2023. The Author(s).
Conflict of interest statement
CJAP has an advisory role for Nordic Pharma. HAM is a co-founder and shareholder of Nicolab.
JS is a member of the
All remaining authors declare no competing interests.
Figures




References
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
