Use of Voice-Based Conversational Artificial Intelligence for Basal Insulin Prescription Management Among Patients With Type 2 Diabetes: A Randomized Clinical Trial
- PMID: 38039007
- PMCID: PMC10692866
- DOI: 10.1001/jamanetworkopen.2023.40232
Use of Voice-Based Conversational Artificial Intelligence for Basal Insulin Prescription Management Among Patients With Type 2 Diabetes: A Randomized Clinical Trial
Abstract
Importance: Optimizing insulin therapy for patients with type 2 diabetes can be challenging given the need for frequent dose adjustments. Most patients receive suboptimal doses and do not achieve glycemic control.
Objective: To examine whether a voice-based conversational artificial intelligence (AI) application can help patients with type 2 diabetes titrate basal insulin at home to achieve rapid glycemic control.
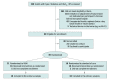
Design, setting, and participants: In this randomized clinical trial conducted at 4 primary care clinics at an academic medical center from March 1, 2021, to December 31, 2022, 32 adults with type 2 diabetes requiring initiation or adjustment of once-daily basal insulin were followed up for 8 weeks. Statistical analysis was performed from January to February 2023.
Interventions: Participants were randomized in a 1:1 ratio to receive basal insulin management with a voice-based conversational AI application or standard of care.
Main outcomes and measures: Primary outcomes were time to optimal insulin dose (number of days needed to achieve glycemic control), insulin adherence, and change in composite survey scores measuring diabetes-related emotional distress and attitudes toward health technology and medication adherence. Secondary outcomes were glycemic control and glycemic improvement. Analysis was performed on an intent-to-treat basis.
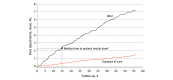
Results: The study population included 32 patients (mean [SD] age, 55.1 [12.7] years; 19 women [59.4%]). Participants in the voice-based conversational AI group more quickly achieved optimal insulin dosing compared with the standard of care group (median, 15 days [IQR, 6-27 days] vs >56 days [IQR, >29.5 to >56 days]; a significant difference in time-to-event curves; P = .006) and had better insulin adherence (mean [SD], 82.9% [20.6%] vs 50.2% [43.0%]; difference, 32.7% [95% CI, 8.0%-57.4%]; P = .01). Participants in the voice-based conversational AI group were also more likely than those in the standard of care group to achieve glycemic control (13 of 16 [81.3%; 95% CI, 53.7%-95.0%] vs 4 of 16 [25.0%; 95% CI, 8.3%-52.6%]; difference, 56.3% [95% CI, 21.4%-91.1%]; P = .005) and glycemic improvement, as measured by change in mean (SD) fasting blood glucose level (-45.9 [45.9] mg/dL [95% CI, -70.4 to -21.5 mg/dL] vs 23.0 [54.7] mg/dL [95% CI, -8.6 to 54.6 mg/dL]; difference, -68.9 mg/dL [95% CI, -107.1 to -30.7 mg/dL]; P = .001). There was a significant difference between the voice-based conversational AI group and the standard of care group in change in composite survey scores measuring diabetes-related emotional distress (-1.9 points vs 1.7 points; difference, -3.6 points [95% CI, -6.8 to -0.4 points]; P = .03).
Conclusions and relevance: In this randomized clinical trial of a voice-based conversational AI application that provided autonomous basal insulin management for adults with type 2 diabetes, participants in the AI group had significantly improved time to optimal insulin dose, insulin adherence, glycemic control, and diabetes-related emotional distress compared with those in the standard of care group. These findings suggest that voice-based digital health solutions can be useful for medication titration.
Trial registration: ClinicalTrials.gov Identifier: NCT05081011.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention. Prevalence of both diagnosed and undiagnosed diabetes. Published September 21, 2022. Accessed February 11, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnose...
-
- Centers for Disease Control and Prevention. Risk factors for diabetes-related complications. Published September 21, 2022. Accessed February 11, 2023. https://www.cdc.gov/diabetes/data/statistics-report/risks-complications....
-
- Centers for Disease Control and Prevention. Type 2 diabetes. Published March 2, 2022. Accessed February 11, 2023. https://www.cdc.gov/diabetes/basics/type2.html
-
- Garber AJ, Handelsman Y, Grunberger G, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26(1):107-139. doi:10.4158/CS-2019-0472 - DOI - PubMed

