Asymmetric Dearomatization of Phthalazines by Anion-Binding Catalysis
- PMID: 38039188
- PMCID: PMC10729020
- DOI: 10.1021/acs.orglett.3c03325
Asymmetric Dearomatization of Phthalazines by Anion-Binding Catalysis
Abstract
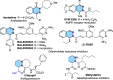
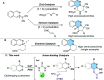
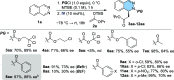
A straightforward methodology for the enantioselective synthesis of 1,2-dihydrophthalazines via dearomatization of phthalazines by anion-binding catalysis has been developed. The process involves the Mannich-type addition of silyl ketene acetals to in situ generated N-acylphthalazinium chlorides using a tert-leucine derived thiourea as a H-bond donor catalyst. Ensuing selective and high-yielding transformations provide appealing dihydro- and tetrahydro-phthalazines, phthalazones, and piperazic acid homologues, en route to biologically relevant molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Pelletier J. C.; Hesson D. P.; Jones K. A.; Costa A.-M. Substituted 1,2-Dihydrophthalazines: Potent, Selective, and Noncompetitive Inhibitors of the AMPA Receptor. J. Med. Chem. 1996, 39, 343–346. 10.1021/jm950740w. - DOI - PubMed
- Welch N. C.; Lin W.; Juranka P. F.; Morris C. E.; Stys P. K. Traditional AMPA receptor antagonists partially block Nav1.6-mediated persistent current. Neuropharmacology 2008, 55, 1165–1171. 10.1016/j.neuropharm.2008.07.015. - DOI - PubMed
-
- Caspers P.; Bury L.; Gaucher B.; Heim J.; Shapiro S.; Siegrist S.; Schmitt-Hoffmann A.; Thenoz L.; Urwyler H. In Vitro and In Vivo Properties of Dihydrophthalazine Antifolates, a Novel Family of Antibacterial Drugs. Antimicrob. Agents Chemother. 2009, 53, 3620–3627. 10.1128/AAC.00377-09. - DOI - PMC - PubMed
- Bowker K. E.; Caspers P.; Gaucher B.; MacGowan A. P. In Vitro Activities of Three New Dihydrofolate Reductase Inhibitors against Clinical Isolates of Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2009, 53, 4949–4952. 10.1128/AAC.00845-09. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

