Highly sensitive single-molecule detection of macromolecule ion beams
- PMID: 38039360
- PMCID: PMC10691769
- DOI: 10.1126/sciadv.adj2801
Highly sensitive single-molecule detection of macromolecule ion beams
Abstract
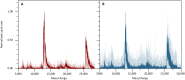
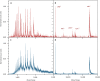
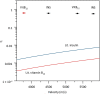
The analysis of proteins in the gas phase benefits from detectors that exhibit high efficiency and precise spatial resolution. Although modern secondary electron multipliers already address numerous analytical requirements, additional methods are desired for macromolecules at energies lower than currently used in post-acceleration detection. Previous studies have proven the sensitivity of superconducting detectors to high-energy particles in time-of-flight mass spectrometry. Here, we demonstrate that superconducting nanowire detectors are exceptionally well suited for quadrupole mass spectrometry and exhibit an outstanding quantum yield at low-impact energies. At energies as low as 100 eV, the sensitivity of these detectors surpasses conventional ion detectors by three orders of magnitude, and they offer the possibility to discriminate molecules by their impact energy and charge. We demonstrate three developments with these compact and sensitive devices, the recording of 2D ion beam profiles, photochemistry experiments in the gas phase, and advanced cryogenic electronics to pave the way toward highly integrated detectors.
Figures




References
-
- W. Paul, Electromagnetic traps for charged and neutral particles. Rev. Mod. Phys. 62, 531–540 (1990).
-
- K. Fehre, D. Trojanowskaja, J. Gatzke, M. Kunitski, F. Trinter, S. Zeller, L. P. H. Schmidt, J. Stohner, R. Berger, A. Czasch, O. Jagutzki, T. Jahnke, R. Dorner, M. S. Schoffler, Absolute ion detection efficiencies of microchannel plates and funnel microchannel plates for multi-coincidence detection. Rev. Sci. Instrum. 89, 045112 (2018). - PubMed
-
- A. Brunelle, P. Chaurand, S. Della-Negra, Y. L. Beyec, E. Parilis, Secondary electron emission yields from a csi surface under impacts of large molecules at low velocities (5x103–7x104 ms–1). Rap. Comm. Mass Spectr. 11, 353–362 (1997).
-
- R. J. Beuhler, L. Friedman, Threshold studies of secondary electron emission induced by macro-ion impact on solid surfaces. Nucl. Instr. Meth. Phys. Res. A 170, 309–315 (1980).
-
- D. Twerenbold, D. Gerber, D. Gritti, Y. Gonin, A. Netuschill, F. Rossel, D. Schenker, J.-L. Vuilleumier, Single molecule detector for mass spectrometry with mass independent detection efficiency. Proteomics 1, 66–69 (2001). - PubMed
LinkOut - more resources
Full Text Sources

