Genetically engineered transfusable platelets using mRNA lipid nanoparticles
- PMID: 38039367
- PMCID: PMC10691771
- DOI: 10.1126/sciadv.adi0508
Genetically engineered transfusable platelets using mRNA lipid nanoparticles
Abstract
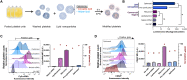
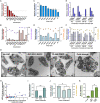
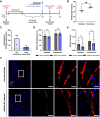
Platelet transfusions are essential for managing bleeding and hemostatic dysfunction and could be expanded as a cell therapy due to the multifunctional role of platelets in various diseases. Creating these cell therapies will require modifying transfusable donor platelets to express therapeutic proteins. However, there are currently no appropriate methods for genetically modifying platelets collected from blood donors. Here, we describe an approach using platelet-optimized lipid nanoparticles containing mRNA (mRNA-LNP) to enable exogenous protein expression in human and rat platelets. Within the library of mRNA-LNP tested, exogenous protein expression did not require nor correlate with platelet activation. Transfected platelets retained hemostatic function and accumulated in regions of vascular damage after transfusion into rats with hemorrhagic shock. We expect this technology will expand the therapeutic potential of platelets.
Figures


References
-
- P. E. J. van der Meijden, J. W. M. Heemskerk, Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 16, 166–179 (2019). - PubMed
-
- X. R. Xu, D. Zhang, B. E. Oswald, N. Carrim, X. Wang, Y. Hou, Q. Zhang, C. Lavalle, T. McKeown, A. H. Marshall, H. Ni, Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 53, 409–430 (2016). - PubMed
-
- J. Johnson, Y.-W. Wu, C. Blyth, G. Lichtfuss, H. Goubran, T. Burnouf, Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 39, 598–612 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

