Early life adversity shapes social subordination and cell type-specific transcriptomic patterning in the ventral hippocampus
- PMID: 38039370
- PMCID: PMC10691768
- DOI: 10.1126/sciadv.adj3793
Early life adversity shapes social subordination and cell type-specific transcriptomic patterning in the ventral hippocampus
Abstract
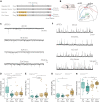
Adverse events in early life can modulate the response to additional stressors later in life and increase the risk of developing psychiatric disorders. The underlying molecular mechanisms responsible for these effects remain unclear. Here, we uncover that early life adversity (ELA) in mice leads to social subordination. Using single-cell RNA sequencing (scRNA-seq), we identified cell type-specific changes in the transcriptional state of glutamatergic and GABAergic neurons in the ventral hippocampus of ELA mice after exposure to acute social stress in adulthood. These findings were reflected by an alteration in excitatory and inhibitory synaptic transmission induced by ELA in response to acute social stress. Finally, enhancing the inhibitory network function through transient diazepam treatment during an early developmental sensitive period reversed the ELA-induced social subordination. Collectively, this study significantly advances our understanding of the molecular, physiological, and behavioral alterations induced by ELA, uncovering a previously unknown cell type-specific vulnerability to ELA.
Figures





References
-
- C. Heim, C. B. Nemeroff, Neurobiology of early life stress: Clinical studies. Semin. Clin. Neuropsychiatry 7, 147–159 (2002). - PubMed
-
- P. E. Mullen, J. L. Martin, J. C. Anderson, S. E. Romans, G. P. Herbison, The long-term impact of the physical, emotional, and sexual abuse of children: A community study. Child Abuse Negl. 20, 7–21 (1996). - PubMed
-
- C. B. Nemeroff, Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron 89, 892–909 (2016). - PubMed
-
- R. C. Kessler, K. A. McLaughlin, J. G. Green, M. J. Gruber, N. A. Sampson, A. M. Zaslavsky, S. Aguilar-Gaxiola, A. O. Alhamzawi, J. Alonso, M. Angermeyer, C. Benjet, E. Bromet, S. Chatterji, G. de Girolamo, K. Demyttenaere, J. Fayyad, S. Florescu, G. Gal, O. Gureje, J. M. Haro, C.-Y. Hu, E. G. Karam, N. Kawakami, S. Lee, J.-P. Lépine, J. Ormel, J. Posada-Villa, R. Sagar, A. Tsang, T. B. Ustün, S. Vassilev, M. C. Viana, D. R. Williams, Childhood adversities and adult psychopathology in the who world mental health surveys. Br. J. Psychiatry 197, 378–385 (2010). - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

