Venetoclax-based low intensity therapy in molecular failure of NPM1-mutated AML
- PMID: 38039513
- PMCID: PMC10788851
- DOI: 10.1182/bloodadvances.2023011106
Venetoclax-based low intensity therapy in molecular failure of NPM1-mutated AML
Abstract
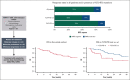
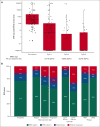
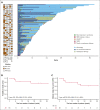
Molecular failure in NPM1-mutated acute myeloid leukemia (AML) inevitably progresses to frank relapse if untreated. Recently published small case series show that venetoclax combined with low-dose cytarabine or azacitidine can reduce or eliminate measurable residual disease (MRD). Here, we report on an international multicenter cohort of 79 patients treated for molecular failure with venetoclax combinations and report an overall molecular response (≥1-log reduction in MRD) in 66 patients (84%) and MRD negativity in 56 (71%). Eighteen of 79 patients (23%) required hospitalization, and no deaths were reported during treatment. Forty-one patients were bridged to allogeneic transplant with no further therapy, and 25 of 41 were MRD negative assessed by reverse transcription quantitative polymerase chain reaction before transplant. Overall survival (OS) for the whole cohort at 2 years was 67%, event-free survival (EFS) was 45%, and in responding patients, there was no difference in survival in those who received a transplant using time-dependent analysis. Presence of FLT3-ITD mutation was associated with a lower response rate (64 vs 91%; P < .01), worse OS (hazard ratio [HR], 2.50; 95% confidence interval [CI], 1.06-5.86; P = .036), and EFS (HR, 1.87; 95% CI, 1.06-3.28; P = .03). Eighteen of 35 patients who did not undergo transplant became MRD negative and stopped treatment after a median of 10 months, with 2-year molecular relapse free survival of 62% from the end of treatment. Venetoclax-based low intensive chemotherapy is a potentially effective treatment for molecular relapse in NPM1-mutated AML, either as a bridge to transplant or as definitive therapy.
© 2024 by The American Society of Hematology. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflict-of-interest disclosure: A.M.-R. reports consultancy or advisory role in Bristol Myers Squibb (BMS), AbbVie, and Kite Gilead; travel grants from Kite Gilead, Roche, Takeda, Janssen, and AbbVie; and speaker fees from AbbVie and Gilead. A.H.W. has served on advisory boards for Novartis, AstraZeneca, Astellas, Janssen, Jazz, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, BMS, and BeiGene; has consulted for AbbVie, Servier, Novartis, Shoreline, and Aculeus; receives research funding to the institution from Novartis, AbbVie, Servier, BMS, Syndax, Astex, AstraZeneca, and Amgen; serves on speaker’s bureaus for AbbVie, Novartis, BMS, Servier, and Astellas; is an employee of the Walter and Eliza Hall Institute (WEHI), and WEHI receives milestone and royalty payments related to the development of venetoclax; current and past employees of WEHI may be eligible for financial benefits related to these payments, and A.H.W. receives such a financial benefit. M. Jädersten has received institutional support from AbbVie for arranging educational webinars and has served on advisory boards for AbbVie. S.K. has served on advisory boards for Astellas, Jazz, AbbVie, Servier, and Novartis; speaker’s bureau of Astellas, Jazz, and Novartis; and research funding from Novartis. D.T.K. received consulting/advisory fees from AbbVie, Atheneum, and Astellas Pharma. V.M. has provided consultancy and received speaker honorarium from AbbVie, Jazz, Novartis, and Pfizer, and educational grants from Astellas and Takeda. N.O. has served on advisory boards for Takeda and Jazz; has consulted for AbbVie, Astellas, BMS, and Servier; and has received support for conference registration/accommodation/travel costs from AbbVie, Jazz, Pfizer, Servier, and Takeda. A.S.R. has provided consultancy to AbbVie and received travel grants from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures


References
-
- Balsat M, Renneville A, Thomas X, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French association group. J Clin Oncol. 2017;35(2):185–193. - PubMed
-
- Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–2716. - PubMed
-
- Kapp-Schwoerer S, Weber D, Corbacioglu A, et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: results from the AMLSG 09-09 trial. Blood. 2020;136(26):3041–3050. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

