ALK inhibitors increase ALK expression and sensitize neuroblastoma cells to ALK.CAR-T cells
- PMID: 38039964
- PMCID: PMC10793157
- DOI: 10.1016/j.ccell.2023.11.004
ALK inhibitors increase ALK expression and sensitize neuroblastoma cells to ALK.CAR-T cells
Abstract
Selection of the best tumor antigen is critical for the therapeutic success of chimeric antigen receptor (CAR) T cells in hematologic malignancies and solid tumors. The anaplastic lymphoma kinase (ALK) receptor is expressed by most neuroblastomas while virtually absent in most normal tissues. ALK is an oncogenic driver in neuroblastoma and ALK inhibitors show promising clinical activity. Here, we describe the development of ALK.CAR-T cells that show potent efficacy in monotherapy against neuroblastoma with high ALK expression without toxicity. For neuroblastoma with low ALK expression, combination with ALK inhibitors specifically potentiates ALK.CAR-T cells but not GD2.CAR-T cells. Mechanistically, ALK inhibitors impair tumor growth and upregulate the expression of ALK, thereby facilitating the activity of ALK.CAR-T cells against neuroblastoma. Thus, while neither ALK inhibitors nor ALK.CAR-T cells will likely be sufficient as monotherapy in neuroblastoma with low ALK density, their combination specifically enhances therapeutic efficacy.
Keywords: ALK; adoptive T cell therapy; cell engineering; cellular immunity; chimeric antigen receptors; lorlatinib; neuroblastoma; solid tumors; tyrosine kinase inhibitors.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.A. is an employee of Celldex Therapeutics, a company that developed anti-ALK antibodies for therapeutic applications. E.B., W.-T.T., and R.C. filed a patent related to this work covering the development of ALK.CAR-T cells.
Figures
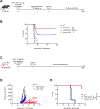


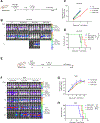
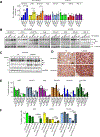



References
-
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. (2008). Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature medicine 14, 1264–1270. 10.1038/nm.1882. - DOI - PMC - PubMed
-
- Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, Liu H, Wu MF, Mei Z, Gee A, et al. (2017). CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Molecular therapy : the journal of the American Society of Gene Therapy 25, 2214–2224. 10.1016/j.ymthe.2017.05.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

