Fgf signalling is required for gill slit formation in the skate, Leucoraja erinacea
- PMID: 38040078
- PMCID: PMC11195640
- DOI: 10.1016/j.ydbio.2023.11.008
Fgf signalling is required for gill slit formation in the skate, Leucoraja erinacea
Abstract
The gill slits of fishes develop from an iterative series of pharyngeal endodermal pouches that contact and fuse with surface ectoderm on either side of the embryonic head. We find in the skate (Leucoraja erinacea) that all gill slits form via a stereotypical sequence of epithelial interactions: 1) endodermal pouches approach overlying surface ectoderm, with 2) focal degradation of ectodermal basement membranes preceding endoderm-ectoderm contact; 3) endodermal pouches contact and intercalate with overlying surface ectoderm, and finally 4) perforation of a gill slit occurs by epithelial remodelling, without programmed cell death, at the site of endoderm-ectoderm intercalation. Skate embryos express Fgf8 and Fgf3 within developing pharyngeal epithelia during gill slit formation. When we inhibit Fgf signalling by treating skate embryos with the Fgf receptor inhibitor SU5402 we find that endodermal pouch formation, basement membrane degradation and endodermal-ectodermal intercalation are unaffected, but that epithelial remodelling and gill slit perforation fail to occur. These findings point to a role for Fgf signalling in epithelial remodelling during gill slit formation in the skate and, more broadly, to an ancestral role for Fgf signalling during pharyngeal pouch epithelial morphogenesis in vertebrate embryos.
Keywords: Ectoderm; Endoderm; Fgf3; Fgf8; Gill slit; Pharyngeal pouch; Skate.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
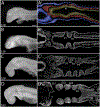

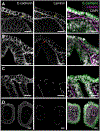




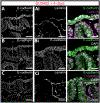
Similar articles
-
Endodermal/ectodermal interfaces during pharyngeal segmentation in vertebrates.J Anat. 2014 Nov;225(5):479-91. doi: 10.1111/joa.12234. Epub 2014 Sep 8. J Anat. 2014. PMID: 25201771 Free PMC article.
-
The conundrum of pharyngeal teeth origin: the role of germ layers, pouches, and gill slits.Biol Rev Camb Philos Soc. 2022 Feb;97(1):414-447. doi: 10.1111/brv.12805. Epub 2021 Oct 13. Biol Rev Camb Philos Soc. 2022. PMID: 34647411 Free PMC article. Review.
-
Ectodermal Wnt signaling, cell fate determination, and polarity of the skate gill arch skeleton.Elife. 2023 Mar 20;12:e79964. doi: 10.7554/eLife.79964. Elife. 2023. PMID: 36940244 Free PMC article.
-
Embryonic origin and serial homology of gill arches and paired fins in the skate, Leucoraja erinacea.Elife. 2020 Nov 17;9:e60635. doi: 10.7554/eLife.60635. Elife. 2020. PMID: 33198887 Free PMC article.
-
Dynamic epithelia of the developing vertebrate face.Curr Opin Genet Dev. 2015 Jun;32:66-72. doi: 10.1016/j.gde.2015.02.003. Epub 2015 Mar 3. Curr Opin Genet Dev. 2015. PMID: 25748249 Free PMC article. Review.
Cited by
-
Less, but More: New Insights From Appendicularians on Chordate Fgf Evolution and the Divergence of Tunicate Lifestyles.Mol Biol Evol. 2025 Jan 6;42(1):msae260. doi: 10.1093/molbev/msae260. Mol Biol Evol. 2025. PMID: 39686543 Free PMC article.
-
Periderm fate and independence of tooth formation are conserved across osteichthyans.Evodevo. 2024 Oct 3;15(1):13. doi: 10.1186/s13227-024-00232-4. Evodevo. 2024. PMID: 39363199 Free PMC article.
References
-
- Abu-Issa R, Smyth G, Smoak I, Yamamura K-I, Meyers EN, 2002. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129, 4613–4625. - PubMed
-
- Ballard WW, Mellinger J, Lechenault H, 1993. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J. Exp. Zool 267, 318–336.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

