Multicenter randomized controlled trial of neoadjuvant chemoradiotherapy alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic adenocarcinoma
- PMID: 38040420
- PMCID: PMC10693876
- DOI: 10.1136/jitc-2023-007586
Multicenter randomized controlled trial of neoadjuvant chemoradiotherapy alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a challenging target for immunotherapy because it has an immunosuppressive tumor microenvironment. Neoadjuvant chemoradiotherapy can increase tumor-infiltrating lymphocyte (TIL) density, which may predict overall survival (OS). We hypothesized that adding programmed cell death protein 1 (PD-1) blockade to chemoradiotherapy would be well tolerated and increase TILs among patients with localized PDAC.
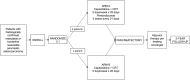
Methods: Patients were randomized 2:1 to Arm A (receiving pembrolizumab plus chemoradiotherapy (capecitabine and external beam radiation)) or Arm B (receiving chemoradiotherapy alone) before anticipated pancreatectomy. Primary endpoints were (1) incidence and severity of adverse events during neoadjuvant therapy and (2) density of TILs in resected tumor specimens. TIL density was assessed using multiplexed immunofluorescence histologic examination.
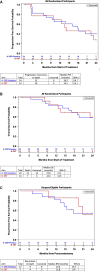
Results: Thirty-seven patients were randomized to Arms A (n=24) and B (n=13). Grade ≥3 adverse events related to neoadjuvant treatment were experienced by 9 (38%) and 4 (31%) patients in Arms A and B, respectively, with one patient experiencing dose-limiting toxicity in Arm A. Seventeen (71%) and 7 (54%) patients in Arms A and B, respectively, underwent pancreatectomy. Median CD8+ T-cell densities in Arms A and B were 67.4 (IQR: 39.2-141.8) and 37.9 (IQR: 22.9-173.4) cells/mm2, respectively. Arms showed no noticeable differences in density of CD8+Ki67+, CD4+, or CD4+FOXP3+ regulatory T cells; M1-like and M2-like macrophages; or granulocytes. Median OS durations were 27.8 (95% CI: 17.1 to NR) and 24.3 (95% CI: 12.6 to NR) months for Arms A and B, respectively.
Conclusions: Adding pembrolizumab to neoadjuvant chemoradiotherapy was safe. However, no convincing effect on CD8+ TILs was observed.
Keywords: Adjuvants, Immunologic; Clinical Trials as Topic; Combined Modality Therapy; Immune Checkpoint Inhibitors; Immunotherapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This clinical trial was funded by Merck as an investigator-initiated clinical trial (PI: OR). SKD received research funding unrelated to this project from Eli Lilly and Company, Novartis Pharmaceuticals, Genocea, and Bristol-Myers Squibb and is a founder, science advisory board member and equity holder in Kojin. BMW has received consulting fees from Celgene, GRAIL, and Mirati, and research funding from Celgene, Eli Lilly, Novartis, and Revolution Medicines unrelated to this work. TSB-S received research Funding (to institution) unrelated to this project from Agios, Arys, Arcus, Atreca, Boston Biomedical, Bayer, Eisai, Celgene, Lilly, Ipsen, Clovis, Seattle Genetics, Genentech, Novartis, Mirati, Merus, Abgenomics, Incyte, Pfizer, BMS. He received consulting fees to institution from Servier, Ipsen, Arcus, Pfizer, Seattle Genetics, Bayer, Genentech, Incyte, Eisai, Merus, Merck KGA and Merck and to self from Stemline, AbbVie, Blueprint Medicines, Boehringer Ingelheim, Janssen, Daiichi Sankyo, Natera, TreosBio, Celularity, Caladrius Biosciences, Exact Science, Sobi, Beigene, Kanaph, AstraZeneca, Deciphera, Zai Labs, Exelixis, Foundation Medicine and Sanofi. GlaxoSmithKline. TB serves on the IDMC/DSMB for the Valley Hospital, Fibrogen, Suzhou Kintor, AstraZeneca, Exelixis, Merck/Eisai, PanCan and 1Globe.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
