Targeting integrin α5 in fibroblasts potentiates colorectal cancer response to PD-L1 blockade by affecting extracellular-matrix deposition
- PMID: 38040421
- PMCID: PMC10693881
- DOI: 10.1136/jitc-2023-007447
Targeting integrin α5 in fibroblasts potentiates colorectal cancer response to PD-L1 blockade by affecting extracellular-matrix deposition
Abstract
Background: One reason patients with cancer cannot benefit from immunotherapy is the lack of immune cell infiltration in tumor tissues. Cancer-associated fibroblasts (CAFs) are emerging as central players in immune regulation that shapes tumor microenvironment (TME). Earlier we reported that integrin α5 was enriched in CAFs in colorectal cancer (CRC), however, its role in TME and cancer immunotherapy remains unclear. Here, we aimed to investigate the role for integrin α5 in fibroblasts in modulating antitumor immunity and therapeutic efficacy combined with checkpoint blockade in CRC.
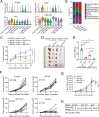
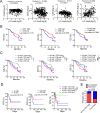
Methods: We analyzed the CRC single-cell RNA sequencing (scRNA-seq) database to define the expression of ITGA5 in CRC tumor stroma. Experimentally, we carried out in vivo mouse tumor xenograft models to confirm the targeting efficacy of combined α5β1 inhibition and anti-Programmed death ligand 1 (PD-L1) blockade and in vitro cell-co-culture assay to investigate the role of α5 in fibroblasts in affecting T-cell activity. Clinically, we analyzed the association between α5 expression and infiltrating T cells and evaluated their correlation with patient survival and immunotherapy prognosis in CRC.
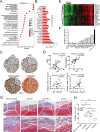
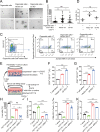
Results: We revealed that ITGA5 was enriched in FAP-CAFs. Both ITGA5 knockout fibroblasts and therapeutic targeting of α5 improved response to anti-PD-L1 treatment in mouse subcutaneous tumor models. Mechanistically, these treatments led to increased tumor-infiltrating CD8+ T cells. Furthermore, we found that α5 in fibroblasts correlated with extracellular matrix (ECM)-related genes and affected ECM deposition in CRC tumor stroma. Both in vivo analysis and in vitro culture and cell killing experiment showed that ECM proteins and α5 expression in fibroblasts influence T-cell infiltration and activity. Clinically, we confirmed that high α5 expression was associated with fewer CD3+ T and CD8+ T cells, and tissues with low α5 and high CD3+ T levels correlated with better patient survival and immunotherapy response in a CRC cohort with 29 patients.
Conclusions: Our study identified a role for integrin α5 in fibroblasts in modulating antitumor immunity by affecting ECM deposition and showed therapeutic efficacy for combined α5β1 inhibition and PD-L1 blockade in CRC.
Keywords: Biomarkers, Tumor; CD8-Positive T-Lymphocytes; Immunomodulation; Immunotherapy; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
