Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: insights from the BE GONE trial
- PMID: 38040541
- PMCID: PMC10755114
- DOI: 10.1016/j.ebiom.2023.104873
Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: insights from the BE GONE trial
Abstract
Background: Accessible prebiotic foods hold strong potential to jointly target gut health and metabolic health in high-risk patients. The BE GONE trial targeted the gut microbiota of obese surveillance patients with a history of colorectal neoplasia through a straightforward bean intervention.
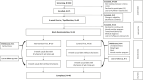
Methods: This low-risk, non-invasive dietary intervention trial was conducted at MD Anderson Cancer Center (Houston, TX, USA). Following a 4-week equilibration, patients were randomized to continue their usual diet without beans (control) or to add a daily cup of study beans to their usual diet (intervention) with immediate crossover at 8-weeks. Stool and fasting blood were collected every 4 weeks to assess the primary outcome of intra and inter-individual changes in the gut microbiome and in circulating markers and metabolites within 8 weeks. This study was registered on ClinicalTrials.gov as NCT02843425, recruitment is complete and long-term follow-up continues.
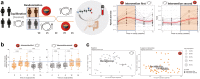
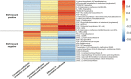
Findings: Of the 55 patients randomized by intervention sequence, 87% completed the 16-week trial, demonstrating an increase on-intervention in diversity [n = 48; linear mixed effect and 95% CI for inverse Simpson index: 0.16 (0.02, 0.30); p = 0.02] and shifts in multiple bacteria indicative of prebiotic efficacy, including increased Faecalibacterium, Eubacterium and Bifidobacterium (all p < 0.05). The circulating metabolome showed parallel shifts in nutrient and microbiome-derived metabolites, including increased pipecolic acid and decreased indole (all p < 0.002) that regressed upon returning to the usual diet. No significant changes were observed in circulating lipoproteins within 8 weeks; however, proteomic biomarkers of intestinal and systemic inflammatory response, fibroblast-growth factor-19 increased, and interleukin-10 receptor-α decreased (p = 0.01).
Interpretation: These findings underscore the prebiotic and potential therapeutic role of beans to enhance the gut microbiome and to regulate host markers associated with metabolic obesity and colorectal cancer, while further emphasizing the need for consistent and sustainable dietary adjustments in high-risk patients.
Funding: This study was funded by the American Cancer Society.
Keywords: Colorectal cancer; Dry beans; Gut microbiota; Inflammation; Metabolites; Obesity; Prebiotic; Proteomic biomarkers.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Study beans were independently purchased with funds from the Dry Bean Health Research Program, a peer-reviewed incentive award (to CRD) created by the Northarvest Bean Growers Association, Communique Inc. to identify and encourage researchers that apply for NIH-funding to support studies on beans and human health.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

