A rapid and stable spontaneous reprogramming system of Spermatogonial stem cells to Pluripotent State
- PMID: 38041111
- PMCID: PMC10693117
- DOI: 10.1186/s13578-023-01150-z
A rapid and stable spontaneous reprogramming system of Spermatogonial stem cells to Pluripotent State
Abstract
Background: The scarcity of pluripotent stem cells poses a major challenge to the clinical application, given ethical and biosafety considerations. While germline stem cells commit to gamete differentiation throughout life, studies demonstrated the spontaneous acquisition of pluripotency by spermatogonial stem cells (SSCs) from neonatal testes at a low frequency (1 in 1.5 × 107). Notably, this process occurs without exogenous oncogenes or chemical supplementation. However, while knockout of the p53 gene accelerates the transformation of SSCs, it also increases risk and hampers their clinical use.
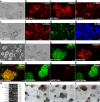
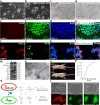
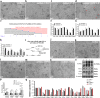
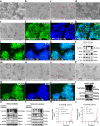
Results: We report a transformation system that efficiently and stably convert SSCs into pluripotent stem cells around 10 passages with the morphology similar to that of epiblast stem cells, which convert to embryonic stem (ES) cell-like colonies after change with ES medium. Epidermal growth factor (EGF), leukemia inhibitory factor (LIF) and fresh mouse embryonic fibroblast feeder (MEF) are essential for transformation, and addition of 2i (CHIR99021 and PD0325901) further enhanced the pluripotency. Transcriptome analysis revealed that EGF activated the RAS signaling pathway and inhibited p38 to initiate transformation, and synergically cooperated with LIF to promote the transformation.
Conclusion: This system established an efficient and safe resource of pluripotent cells from autologous germline, and provide new avenues for regenerative medicine and animal cloning.
Keywords: Cell fate; Feeder layer; Germline; Pluripotency; Reprogramming.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Analysis of chromatin accessibility in p53 deficient spermatogonial stem cells for high frequency transformation into pluripotent state.Cell Prolif. 2022 Mar;55(3):e13195. doi: 10.1111/cpr.13195. Epub 2022 Feb 4. Cell Prolif. 2022. PMID: 35119145 Free PMC article.
-
Characterization of Oct4-GFP spermatogonial stem cell line and its application in the reprogramming studies.J Cell Biochem. 2013 Apr;114(4):920-8. doi: 10.1002/jcb.24431. J Cell Biochem. 2013. PMID: 23097321
-
Timely stimulation of early embryo promotes the acquisition of pluripotency.Cytometry A. 2022 Aug;101(8):682-691. doi: 10.1002/cyto.a.24551. Epub 2022 Mar 25. Cytometry A. 2022. PMID: 35332996
-
Plasticity of spermatogonial stem cells.Asian J Androl. 2015 May-Jun;17(3):355-9. doi: 10.4103/1008-682X.148072. Asian J Androl. 2015. PMID: 25677134 Free PMC article. Review.
-
[Generation and application of pluripotent stem cells from spermatogonial stem cells].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011 Feb;28(1):208-12. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011. PMID: 21485215 Review. Chinese.
Cited by
-
SMAD3 and HIF-1α orchestrate metabolic transition to aerobic glycolysis as a critical prerequisite for spontaneous reprogramming of spermatogonial stem cells.Stem Cell Res Ther. 2025 Jul 28;16(1):411. doi: 10.1186/s13287-025-04541-w. Stem Cell Res Ther. 2025. PMID: 40721814 Free PMC article.
-
Unraveling the Significance of Nanog in the Generation of Embryonic Stem-like Cells from Spermatogonia Stem Cells: A Combined In Silico Analysis and In Vitro Experimental Approach.Int J Mol Sci. 2024 Apr 29;25(9):4833. doi: 10.3390/ijms25094833. Int J Mol Sci. 2024. PMID: 38732061 Free PMC article.
-
From unipotency to pluripotency: deciphering protein networks and signaling pathways in the generation of embryonic stem-like cells from murine spermatogonial stem cells.BMC Genomics. 2025 Apr 30;26(1):426. doi: 10.1186/s12864-025-11612-y. BMC Genomics. 2025. PMID: 40307702 Free PMC article.
References
-
- Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120(11):3197–204. doi: 10.1242/dev.120.11.3197. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

