Molecular responses of agroinfiltrated Nicotiana benthamiana leaves expressing suppressor of silencing P19 and influenza virus-like particles
- PMID: 38041470
- PMCID: PMC11022802
- DOI: 10.1111/pbi.14247
Molecular responses of agroinfiltrated Nicotiana benthamiana leaves expressing suppressor of silencing P19 and influenza virus-like particles
Abstract
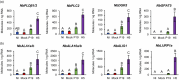
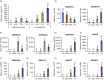
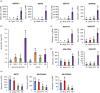
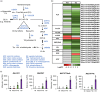
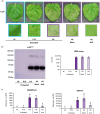
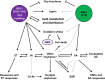
The production of influenza vaccines in plants is achieved through transient expression of viral hemagglutinins (HAs), a process mediated by the bacterial vector Agrobacterium tumefaciens. HA proteins are then produced and matured through the secretory pathway of plant cells, before being trafficked to the plasma membrane where they induce formation of virus-like particles (VLPs). Production of VLPs unavoidably impacts plant cells, as do viral suppressors of RNA silencing (VSRs) that are co-expressed to increase recombinant protein yields. However, little information is available on host molecular responses to foreign protein expression. This work provides a comprehensive overview of molecular changes occurring in Nicotiana benthamiana leaf cells transiently expressing the VSR P19, or co-expressing P19 and an influenza HA. Our data identifies general responses to Agrobacterium-mediated expression of foreign proteins, including shutdown of chloroplast gene expression, activation of oxidative stress responses and reinforcement of the plant cell wall through lignification. Our results also indicate that P19 expression promotes salicylic acid (SA) signalling, a process dampened by co-expression of the HA protein. While reducing P19 level, HA expression also induces specific signatures, with effects on lipid metabolism, lipid distribution within membranes and oxylipin-related signalling. When producing VLPs, dampening of P19 responses thus likely results from lower expression of the VSR, crosstalk between SA and oxylipin pathways, or a combination of both outcomes. Consistent with the upregulation of oxidative stress responses, we finally show that reduction of oxidative stress damage through exogenous application of ascorbic acid improves plant biomass quality during production of VLPs.
Keywords: Nicotiana benthamiana; Influenza hemagglutinin; Plant immunity; Plant molecular farming; Transient Agrobacterium‐mediated expression; Virus‐like particles.
© 2023 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
At the time of this work, L.P.H., R.T., F.P.G., M.E.P., E.R., M.A.C., P.O.L and M.A.D. were employees of Medicago Inc. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


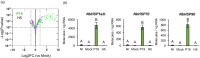



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

