Role of the glymphatic system and perivascular spaces as a potential biomarker for post-stroke epilepsy
- PMID: 38041607
- PMCID: PMC10839409
- DOI: 10.1002/epi4.12877
Role of the glymphatic system and perivascular spaces as a potential biomarker for post-stroke epilepsy
Abstract
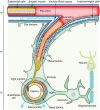
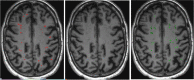
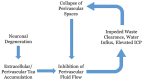
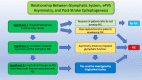
Stroke is one of the most common causes of acquired epilepsy, which can also result in disability and increased mortality rates particularly in elderly patients. No preventive treatment for post-stroke epilepsy is currently available. Development of such treatments has been greatly limited by the lack of biomarkers to reliably identify high-risk patients. The glymphatic system, including perivascular spaces (PVS), is the brain's waste clearance system, and enlargement or asymmetry of PVS (ePVS) is hypothesized to play a significant role in the pathogenesis of several neurological conditions. In this article, we discuss potential mechanisms for the role of perivascular spaces in the development of post-stroke epilepsy. Using advanced MR-imaging techniques, it has been shown that there is asymmetry and impairment of glymphatic function in the setting of ischemic stroke. Furthermore, studies have described a dysfunction of PVS in patients with different focal and generalized epilepsy syndromes. It is thought that inflammatory processes involving PVS and the blood-brain barrier, impairment of waste clearance, and sustained hypertension affecting the glymphatic system during a seizure may play a crucial role in epileptogenesis post-stroke. We hypothesize that impairment of the glymphatic system and asymmetry and dynamics of ePVS in the course of a stroke contribute to the development of PSE. Automated ePVS detection in stroke patients might thus assist in the identification of high-risk patients for post-stroke epilepsy trials. PLAIN LANGUAGE SUMMARY: Stroke often leads to epilepsy and is one of the main causes of epilepsy in elderly patients, with no preventative treatment available. The brain's waste removal system, called the glymphatic system which consists of perivascular spaces, may be involved. Enlargement or asymmetry of perivascular spaces could play a role in this and can be visualised with advanced brain imaging after a stroke. Detecting enlarged perivascular spaces in stroke patients could help identify those at risk for post-stroke epilepsy.
Keywords: blood-brain barrier; glymphatic system; neuroinflammation; perivascular spaces; post-stroke epilepsy.
© 2023 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The author Gernot Hlauschek has served as a lecturer for Eisai, unrelated to this study. The author Morten Ingvar Lossius has served as a paid consultant and lecturer for Eisai, UCB, and Arvelle Therapeutics, unrelated to this study. None of the other authors has any conflict of interest to disclose.
Figures

Similar articles
-
Asymmetric distribution of enlarged perivascular spaces in centrum semiovale may be associated with epilepsy after acute ischemic stroke.CNS Neurosci Ther. 2022 Mar;28(3):343-353. doi: 10.1111/cns.13786. Epub 2022 Jan 3. CNS Neurosci Ther. 2022. PMID: 34981639 Free PMC article.
-
Automated Methods for Detecting and Quantitation of Enlarged Perivascular spaces on MRI.J Magn Reson Imaging. 2023 Jan;57(1):11-24. doi: 10.1002/jmri.28369. Epub 2022 Jul 22. J Magn Reson Imaging. 2023. PMID: 35866259 Free PMC article. Review.
-
Enlarged perivascular spaces, neuroinflammation and neurological dysfunction in NMOSD patients.Front Immunol. 2022 Sep 29;13:966781. doi: 10.3389/fimmu.2022.966781. eCollection 2022. Front Immunol. 2022. PMID: 36248814 Free PMC article.
-
Aqueductal CSF stroke volume is associated with the burden of perivascular space enlargement in chronic adult hydrocephalus.Sci Rep. 2024 Jun 5;14(1):12966. doi: 10.1038/s41598-024-63926-8. Sci Rep. 2024. PMID: 38839864 Free PMC article.
-
Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology.Neuroradiology. 2021 Oct;63(10):1581-1597. doi: 10.1007/s00234-021-02718-7. Epub 2021 May 21. Neuroradiology. 2021. PMID: 34019111 Free PMC article. Review.
Cited by
-
Impaired glymphatic system in patent foramen ovale based on diffusion tensor imaging analysis along the perivascular space.Quant Imaging Med Surg. 2025 Apr 1;15(4):2987-2999. doi: 10.21037/qims-24-1963. Epub 2025 Mar 19. Quant Imaging Med Surg. 2025. PMID: 40235821 Free PMC article.
-
Glymphatic system dysfunction in cerebral infarction: advances and perspectives based on DTI-derived ALPS measures.Am J Transl Res. 2025 Mar 15;17(3):1630-1642. doi: 10.62347/OQRE2088. eCollection 2025. Am J Transl Res. 2025. PMID: 40226042 Free PMC article. Review.
-
The Association of Epileptic Seizures after Acute Ischemic Stroke with Cerebral Cortical Involvement and Electroencephalographic Changes.Medicina (Kaunas). 2024 May 6;60(5):768. doi: 10.3390/medicina60050768. Medicina (Kaunas). 2024. PMID: 38792951 Free PMC article.
-
Glymphatic system in neurological disorders and implications for brain health.Front Neurol. 2025 Feb 5;16:1543725. doi: 10.3389/fneur.2025.1543725. eCollection 2025. Front Neurol. 2025. PMID: 39974364 Free PMC article. No abstract available.
-
Association of glymphatic system dysfunction with cognitive impairment in temporal lobe epilepsy.Front Aging Neurosci. 2024 Oct 18;16:1459580. doi: 10.3389/fnagi.2024.1459580. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39493279 Free PMC article.
References
-
- Arntz RM, Maaijwee NA, Rutten‐Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, et al. Epilepsy after TIA or stroke in young patients impairs long‐term functional outcome: the FUTURE study. Neurology. 2013;81(22):1907–1913. - PubMed
-
- Beretta S, Carone D, Zanchi C, Bianchi E, Pirovano M, Trentini C, et al. Long‐term applicability of the new ILAE definition of epilepsy. Results from the PRO‐LONG study. Epilepsia. 2017;58(9):1518–1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

