First-in-human trial of a self-expandable, temporary dilation system for intracranial atherosclerotic disease in patients presenting with acute ischemic stroke
- PMID: 38041666
- PMCID: PMC11671925
- DOI: 10.1136/jnis-2023-020983
First-in-human trial of a self-expandable, temporary dilation system for intracranial atherosclerotic disease in patients presenting with acute ischemic stroke
Abstract
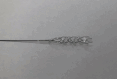
Background: Intracranial atherosclerotic disease (ICAD) significantly contributes to ischemic stroke, especially among Asian populations. Large vessel occlusion (LVO) due to underlying ICAD accounts for 15-35% of acute ischemic stroke cases requiring endovascular therapy. However, the successful recanalization rate of ICAD-related LVO remains lower. The TG dilator is a self-expandable device, temporarily dilating ICAD-related blocked blood vessels.
Objective: To demonstrate TG dilator safety and efficacy for ICAD-related acute ischemic stroke.
Methods: This was a single-arm, open-label, non-randomized, prospective, multicenter, and investigator-initiated trial that involved patients undergoing TG dilator application for acute ischemic stroke caused by ICAD-related LVO or severe stenosis.
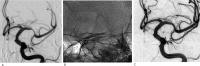
Results: We enrolled 10 patients in this trial between November 2022 and April 2023. The median (IQR) age was 68 (59.3-75.3) years. Before using the dilator, seven patients received stent retriever treatment. All 10 patients were prescribed a loading dose of aspirin with prasugrel. The median application time was 10 (10-12) min. At the end of the procedure, we achieved significant recanalization immediately in all patients. The stenosis/occlusion decreased from 100% (100-100) to 68% (56.3-75.3). No patient experienced recurrent ischemic stroke or reocclusion within 90 days. We achieved a modified Rankin scale score of 0-2 in 8 patients by day 90. We detected no cases of intracranial hemorrhage, equipment failure, distal embolism, vasospasm, dissection, or perforation requiring intervention.
Conclusions: Acute revascularization using the TG dilator on patients with ICAD-related LVO or severe stenosis did not cause any significant adverse event, and consistently improved blood flow at 90 days.
Keywords: Atherosclerosis; Stroke.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TO reports lecturer’s fees from Medtronic, Daiichi-Sankyo, Johnson & Johnson, Terumo, Stryker Japan, Tokai Medical, Otsuka, Takeda, Eisai, Kaneka, Bristol-Myers Squibb, AstraZeneca, Japan Lifeline, Kowa, Nipro, Century Medical, and Idorsia as well as consulting fee for Tokai Medical outside the submitted work. MT reports lecturer's fees from Stryker, Johnson & Johnson, Daiichi-Sankyo, and Asahi-Intec outside the submitted work. HY reports research grants from Bristol-Myers Squibb; lecturer’s fees from Stryker, Medtronic, Johnson & Johnson, Bayer, Daiichi-Sankyo, Bristol-Myers Squibb, and Otsuka Pharmaceutical; advisory board for Daiichi-Sankyo outside the submitted work. KA reports lecturer’s fees from Ono Pharmaceutical, Daiichi-Sankyo, Idorsia, and Medtronic outside the submitted work. AI reports lecturer’s fees from Medtronic, Stryker, Terumo, Kaneka, Johson & Johnson, Asahi-Intec, Abbot vascular, Medicos Hirata, and Daiichi-Sankyo; a research grant from Fuji Film; consulting fees from Medtronic and Terumo outside the submitted work. HI reports lecturer's fees from Medtronic, Stryker, Johnson & Johnson, Terumo, Daiichi-Sankyo, and Asahi-Intec outside the submitted work. SYo reports research grants from Stryker, Siemens Healthineers, Bristol-Myers Squibb, Sanofi, Eisai, Daiichi-Sankyo, Teijin Pharma, Chugai Pharmaceutical, HEALIOS, Asahi Kasei Medical, Kowa, and CSL Behring; lecturer’s fees from Stryker, Medtronic, Johnson & Johnson, Kaneka, Terumo, Biomedical Solutions, Boehringer-Ingelheim, Daiichi-Sankyo, Bayer, and Bristol-Meyers Squibb outside the submitted work. NS reports research grants from Biomedical Solutions, Medtronic, and Terumo; lecturer’s fees from Asahi-Intec, Biomedical Solutions, Daiichi-Sankyo, Kaneka, Medtronic, and Terumo; membership on the advisory boards for Johnson & Johnson, Medtronic, and Terumo outside the submitted work. ST reports consulting fees from Cerenovus, Stryker, Rapid Medical, Phenox, Medtronic, Microvention, NB Medical, NV MedTech, Gravity Medical, Century Medical, and Kaneka Medix outside the submitted work and a consulting fee from T.G. Medical. The other authors have no personal or financial interest in any of the materials or devices described in this article.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous