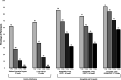The Role of Lifestyle Modification with Second-Generation Anti-obesity Medications: Comparisons, Questions, and Clinical Opportunities
- PMID: 38041774
- PMCID: PMC10748770
- DOI: 10.1007/s13679-023-00534-z
The Role of Lifestyle Modification with Second-Generation Anti-obesity Medications: Comparisons, Questions, and Clinical Opportunities
Abstract
Purpose of review: This review examines lifestyle modification for obesity management with the goal of identifying treatment components that could support the use of a new generation of anti-obesity medications (AOMs).
Recent findings: Semaglutide reliably reduces baseline body weight by approximately 15% at 68 weeks, in contrast to 5-10% for lifestyle modification. Tirzepatide induces mean losses as great as 20.9%. Both medications reduce energy intake by markedly enhancing satiation and decreasing hunger, and they appear to lessen the need for traditional cognitive and behavioral strategies (e.g., monitoring food intake) to achieve calorie restriction. Little, however, is known about whether patients who lose weight with these AOMs adopt healthy diet and activity patterns needed to optimize body composition, cardiometabolic health, and quality of life. When used with the new AOMs, the focus of lifestyle modification is likely to change from inducing weight loss (through calorie restriction) to facilitating patients' adoption of dietary and activity patterns that will promote optimal changes in body composition and overall health.
Keywords: Body composition; Cardiometabolic health; Lifestyle intervention; Obesity; Pharmacotherapy; Weight loss.
© 2023. The Author(s).
Conflict of interest statement
Thomas Wadden serves on scientific advisory boards for Novo Nordisk and WW (Weight Watchers) and has received grant support, on behalf of the University of Pennsylvania, from Eli Lilly, Epitomee Medical, and Novo Nordisk. Ariana Chao has served as a consultant to Eli Lilly and received grant support, on behalf of the University of Pennsylvania, from Eli Lilly and WW (Weight Watchers). Jena Tronieri has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. Anastassia Amaro has served on advisory boards for Fractyl Laboratories, Medality Medical, and Novo Nordisk and has received grant support, on behalf of the University of Pennsylvania, from Altimmune, Eli Lilly, and Fractyl Laboratories. Sharon Leonard has served as a consultant to Eli Lilly and Epitomee Medical. John Jakicic has received grant support, on behalf of Kansas University Medical Center, from Epitomee Medical. The other authors have no disclosures.
Figures
References
-
- •• Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. This pivotal randomized controlled trial of once-weekly semaglutide (2.4 mg) for overweight/obesity reported a mean 14.9% reduction in baseline weight at 68 weeks (compared with 2.4% for placebo) with generally favorable tolerability and safety findings. - PubMed
-
- •• Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16. This pivotal trial of once-weekly tirzepatide (15 mg) for overweight/obesity reported a mean 20.9% reduction in baseline weight at 72 weeks (compared with 3.1% for placebo) with generally favorable tolerability and safety findings. - PubMed
-
- • Jastreboff AM, Kushner RF. New frontiers in obesity treatment: GLP-1 and nascent nutrient stimulated hormone-based therapeutics. Annu Rev Med. 2023;74:125–39. A variety of new drug targets are being explored for overweight/obesity beyond glucagon like polypeptide-1 (GLP-1) receptor agonists (i.e., semaglutide 2.4 mg) and combined glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists (i.e., tirzepatide 15 mg). - PubMed
-
- Wegovy (package insert). Plainsboro, NJ: Novo Nordisk, USA;2021.
-
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl.):51S-210S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials