Epigenetically upregulated NSUN2 confers ferroptosis resistance in endometrial cancer via m5C modification of SLC7A11 mRNA
- PMID: 38042059
- PMCID: PMC10711489
- DOI: 10.1016/j.redox.2023.102975
Epigenetically upregulated NSUN2 confers ferroptosis resistance in endometrial cancer via m5C modification of SLC7A11 mRNA
Abstract
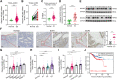
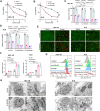
Endometrial cancer (EC) is a prevalent gynecological malignancy worldwide, and 5-methylcytosine (m5C) modification of mRNA is a crucial epigenetic modification associated with the development and occurrence of several cancers. However, the precise function of m5C modification in EC remains elusive. This study aimed to investigate the expression and clinical significance of the primary m5C modification writer, NSUN2, in EC. Our findings indicated that NSUN2 exhibited a substantial up-regulation in EC as a result of an epigenetic augmentation in H3K4me3 levels within the promoter region, which was triggered by the down-regulation of KDM5A. Moreover, gain- and loss-of-function experiments revealed the role of NSUN2 in enhancing m5C modification of mRNA, thereby promoting EC cell proliferation. RNA bisulfite sequencing and transcriptomic sequencing were employed to elucidate the involvement of NSUN2 in the regulation of ferroptosis. Subsequent in vitro experiments confirmed that the knockdown of NSUN2 significantly up-regulated the levels of lipid peroxides and lipid ROS in EC cells, thereby augmenting the susceptibility of EC to ferroptosis. Mechanistically, NSUN2 stimulated the m5C modification of SLC7A11 mRNA, and the m5C reader YBX1 exhibited direct recognition and binding to the m5C sites on SLC7A11 mRNA via its internal cold shock domain (CSD), leading to an increase in SLC7A11 mRNA stability and elevated levels of SLC7A11. Additionally, rescue experiments showed that NSUN2 functioned as a suppressor of ferroptosis, which was dependent on SLC7A11. Overall, targeting the NSUN2/SLC7A11 axis inhibited tumor growth by increasing lipid peroxidation and ferroptosis of EC cells both in vitro and in vivo. Therefore, our study provides new insight into the role of NSUN2, suggesting that NSUN2 may serve as a prognostic biomarker and therapeutic target in patients with EC.
Keywords: Endometrial cancer; Ferroptosis; NSUN2; SLC7A11; m(5)C.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Morice P., et al. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

