Nanoparticle-mediated delivery of non-viral gene editing technology to the brain
- PMID: 38042249
- PMCID: PMC10872436
- DOI: 10.1016/j.pneurobio.2023.102547
Nanoparticle-mediated delivery of non-viral gene editing technology to the brain
Abstract
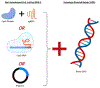
Neurological disorders pose a significant burden on individuals and society, affecting millions worldwide. These disorders, including but not limited to Alzheimer's disease, Parkinson's disease, and Huntington's disease, often have limited treatment options and can lead to progressive degeneration and disability. Gene editing technologies, including Zinc Finger Nucleases (ZFN), Transcription Activator-Like Effector Nucleases (TALEN), and Clustered Regularly Interspaced Short Palindromic Repeats-associated Protein 9 (CRISPR-Cas9), offer a promising avenue for potential cures by targeting and correcting the underlying genetic mutations responsible for neurologic disorders. However, efficient delivery methods are crucial for the successful application of gene editing technologies in the context of neurological disorders. The central nervous system presents unique challenges to treatment development due to the blood-brain barrier, which restricts the entry of large molecules. While viral vectors are traditionally used for gene delivery, nonviral delivery methods, such as nanoparticle-mediated delivery, offer safer alternatives that can efficiently transport gene editing components. Herein we aim to introduce the three main gene editing nucleases as nonviral treatments for neurologic disorders, the delivery barriers associated with brain targeting, and the current nonviral techniques used for brain-specific delivery. We highlight the challenges and opportunities for future research in this exciting and growing field that could lead to blood-brain barrier bypassing therapeutic gene editing.
Keywords: Blood-brain barrier; Gene editing technologies; Genome editing; Non-viral vectors.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Gooch CL, Pracht E & Borenstein AR The burden of neurological disease in the United States: A summary report and call to action. Annals of Neurology 81, 479–484 (2017). - PubMed
-
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR & Anderson DG Non-viral vectors for gene-based therapy. Nature Reviews Genetics 15, 541–555 (2014). - PubMed
-
- Rybicki EP CRISPR–Cas9 strikes out in cassava. Nat Biotechnol 37, 727–728 (2019). - PubMed
-
- Kootstra NA & Verma IM Gene Therapy with Viral Vectors. Annual Review of Pharmacology and Toxicology 43, 413–439 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

