Local fistula injection of allogeneic human amnion epithelial cells is safe and well tolerated in patients with refractory complex perianal Crohn's disease: a phase I open label study with long-term follow up
- PMID: 38042747
- PMCID: PMC10755113
- DOI: 10.1016/j.ebiom.2023.104879
Local fistula injection of allogeneic human amnion epithelial cells is safe and well tolerated in patients with refractory complex perianal Crohn's disease: a phase I open label study with long-term follow up
Abstract
Background: Local fistula injection of mesenchymal stromal/stem cells (MSC) is effective for complex perianal Crohn's fistulas but is also expensive and requires specialised facilities for cell revival before administration. Human amnion epithelial cells (hAEC) are non-MSC cells with therapeutic properties. The primary aim of this study was safety of hAEC therapy. Secondary aims included hAEC efficacy, feasibility of the protocol and impact on quality of life.
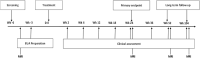
Methods: A phase I open label study of ten adults with active complex Crohn's perianal fistulas refractory to conventional treatment, including anti-tumour necrosis factor alpha therapy, was undertaken. A single dose of hAEC was injected into the fistula tract(s) after surgical closure of the internal opening(s). Study outcomes were assessed at week 24 with follow up for at least 52 weeks.
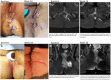
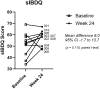
Findings: Local injection of hAEC was safe, well tolerated and the injection procedure was feasible. Complete response occurred in 4 patients, and a partial response in an additional 4 patients. There was a mean reduction in the Perianal Disease Activity Index of 6.5 points (95% CI -9.0 to -4.0, p = 0.0002, paired t-test), modified Van Assche MRI Index of 2.3 points (95% CI -3.9 to -0.6, p = 0.012, paired t-test) and a mean improvement of 15.8 points (95% CI 4.9 to 26.8, p = 0.010, paired t-test) in quality of life using the Short IBD-Questionnaire in complete responders.
Interpretation: Local injection of hAEC therapy for refractory complex perianal fistulising Crohn's disease appears safe, well-tolerated, feasible and demonstrated improvement. Quality of life is improved in those who achieve complete fistula healing.
Funding: This study was funded by competitive research grant funding from the Gastroenterological Society of Australia Seed Grant 2018.
Keywords: Crohn's disease; Human amnion epithelial cells; Perianal fistula; Stem cells.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests A provisional patent (AU2022901083) has been filed in Australia for use of human amnion epithelial cells in perianal fistulising Crohn's disease with RL, CK and GM listed as co-inventors. A company (Exosome Biosciences Pty Ltd 2023) co-owned by the Hudson Institute of Medical Research, Monash University and Monash Health has been established using derivatives of human amnion epithelial cells to treat Crohn's disease. The authors have not received any monetary payments from the company and this company had no role in any part of this study. WS, RL, CK, TCN and GM will participate in a future clinical trial affiliated with Exosome Biosciences. AG has nothing to declare.
Figures


References
-
- Schwartz D.A., Loftus E.V., Jr., Tremaine W.J., et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(4):875–880. - PubMed
-
- Lonnfors S., Vermeire S., Greco M., Hommes D., Bell C., Avedano L. IBD and health-related quality of life -- discovering the true impact. J Crohns Colitis. 2014;8(10):1281–1286. - PubMed
-
- de Groof E.J., Sahami S., Lucas C., Ponsioen C.Y., Bemelman W.A., Buskens C.J. Treatment of perianal fistula in Crohn's disease: a systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Colorectal Dis. 2016;18(7):667–675. - PubMed
-
- Torres J., Bonovas S., Doherty G., et al. ECCO guidelines on therapeutics in crohn's disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. - PubMed
-
- Wewer M.D., Zhao M., Nordholm-Carstensen A., Weimers P., Seidelin J.B., Burisch J. The incidence and disease course of perianal crohn's disease: a Danish Nationwide Cohort Study, 1997-2015. J Crohns Colitis. 2020;15:5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

