Continued improvement in disease manifestations of acid sphingomyelinase deficiency for adults with up to 2 years of olipudase alfa treatment: open-label extension of the ASCEND trial
- PMID: 38042851
- PMCID: PMC10693698
- DOI: 10.1186/s13023-023-02983-0
Continued improvement in disease manifestations of acid sphingomyelinase deficiency for adults with up to 2 years of olipudase alfa treatment: open-label extension of the ASCEND trial
Abstract
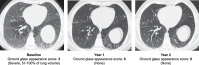
Background: Olipudase alfa is a recombinant human acid sphingomyelinase enzyme replacement therapy for non-central-nervous-system manifestations of acid sphingomyelinase deficiency (ASMD). The ASCEND randomized placebo-controlled trial in adults with ASMD demonstrated reductions in sphingomyelin storage, organomegaly, interstitial lung disease and impaired diffusion capacity of the lung (DLCO), during the first year of olipudase alfa treatment. In an ongoing open-label extension of the ASCEND trial, individuals in the placebo group crossed over to olipudase alfa, and those in the olipudase alfa group continued treatment.
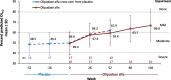
Results: Thirty-five of 36 participants continued in the extension trial, and 33 completed year 2. Change-from-baseline results are presented as least-square mean percent change ± SEM. Improvements in the cross-over group after 1 year of treatment paralleled those of the olipudase alfa group from the primary analysis, while clinical improvement continued for those receiving olipudase alfa for 2 years. In the cross-over group, percent-predicted DLCO increased by 28.0 ± 6.2%, spleen volume decreased by 36.0 ± 3.0% and liver volume decreased by 30.7 ± 2.5%. For those with 2 years of olipudase alfa treatment, the percent predicted DLCO increased by 28.5 ± 6.2%, spleen volume decreased by 47.0 ± 2.7%, and liver volume decreased by 33.4 ± 2.2%. Lipid profiles and elevated liver transaminase levels improved or normalized by 1 year and remained stable through 2 years of treatment. Overall, 99% of treatment-emergent adverse events were mild or moderate, with one treatment-related serious adverse event (extrasystoles; previously documented cardiomyopathy). No individual discontinued due to an adverse event.
Conclusion: Treatment with olipudase alfa is well tolerated and reduces manifestations of chronic ASMD with sustained efficacy. Trial registration NCT02004691 registered 9 December 2013, https://clinicaltrials.gov/ct2/show/NCT02004691.
Keywords: Acid sphingomyelinase deficiency; Dose escalation; Lung diffusing capacity; Niemann–Pick type A/B; Niemann–Pick type B; Organomegaly; Recombinant human acid sphingomyelinase.
© 2023. The Author(s).
Conflict of interest statement
MW: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa and has received travel reimbursement and consulting fees from Sanofi. AB: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa, received honoraria for lectures, advisory boards, meetings, and travel support from Sanofi and Takeda Shire. RCG: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. RG: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa; has received honoraria, consulting fees, speaker fees, and travel reimbursement from Sanofi. NBG: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. JBH: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa; has received speaker fees from Sanofi. CH: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. TI: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. RL: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa; has received consulting fees and travel reimbursement from Sanofi. OL: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. PM: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa and has received consulting fees, speaker fees, and travel reimbursement from Sanofi. EM: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa; has received consulting fees and honoraria from Sanofi. MS: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. ES: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. MT: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. JV: Principal Investigator in the Sanofi sponsored ASCEND trial of olipudase alfa. BLT, AY, NMA, YK, and MK were/are employees of Sanofi (or were at the time of the study) and own stock in the company.
Figures