Effectiveness and safety of everolimus treatment in patients with tuberous sclerosis complex in real-world clinical practice
- PMID: 38042867
- PMCID: PMC10693167
- DOI: 10.1186/s13023-023-02982-1
Effectiveness and safety of everolimus treatment in patients with tuberous sclerosis complex in real-world clinical practice
Abstract
Background: The randomised double-blinded placebo-controlled EXIST-1-3 studies have showed everolimus effective with adverse effects reported as acceptable in treatment of symptoms in patients with tuberous sclerosis complex (TSC), although evidence of outcomes in clinical practice remains limited. This study aimed to investigate, in clinical practice, the effectiveness and safety of everolimus for epilepsy, renal angiomyolipoma (rAML), and subependymal giant cell astrocytoma (SEGA) in patients with TSC.
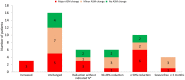
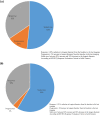
Results: The study included 64 patients with TSC (median age: 19, range 0.9-54 years) receiving everolimus treatment (Norway: n = 35; Denmark: n = 29). Among 45 patients with epilepsy, 14 (31%) were responders experiencing ≥ 50% reduction in seizure frequency in the last 3 months of treatment compared with the last 3 months before treatment. Nineteen (42%) patients changed their anti-seizure medications (ASMs). Responders were more common among patients < 18 years (46%) than among patients ≥ 18 years (14%, p = 0.03). In 29 patients with rAML, everolimus reduced (≥ 30% decrease) and stabilized (< 20% increase, ≤ 30% decrease) longest diameter of rAML in 38% and 59%, respectively, after a mean treatment duration of 37 months. SEGA volume was reduced in three patients by 71%, 43%, and 48% after 39, 34, and 82 months. Adverse effects were reported in 61 of 64 patients (95%) after a median treatment duration of 31 months (range 0-106), with oral ulceration/stomatitis (63%) and upper respiratory tract infections (38%) being the most common. The most common laboratory abnormalities were increased cholesterol (41%), anaemia (30%), and leucopoenia (25%). Grade 3-4 adverse effects were reported in 36% of cases, and life-threatening conditions were reported in two patients. Nine patients discontinued everolimus treatment.
Conclusions: Seizure reduction in this study sample was consistent with results from EXIST, but might be lower than expected, given that changes in concomitant ASMs are part of clinical practice. Seizure reduction was associated with younger age. As with EXIST, everolimus reduced or stabilised rAML size in most patients. SEGA volume was reduced in all three patients. Close follow-up is needed for this group, especially for children and patients who may not be able to report adverse effects.
Keywords: Adverse events; Epilepsy; Everolimus; Renal angiomyolipoma; Sirolimus; Subependymal giant cell astrocytoma; Tuberous sclerosis complex; mTOR inhibitor.
© 2023. The Author(s).
Conflict of interest statement
Ine Cockerell gave an unpaid lecture to UCB Nordic. Jakob Christensen has received honoraria from serving on the scientific advisory board of UCB Nordic and Eisai AB, received honoraria for giving lectures from UCB Nordic and Eisai AB, and received funding for a trip from UCB Nordic. Cecilie Johannessen Landmark has received advisory board/speaker’s honoraria from Angelini, Eisai, Jazz, and UCB Pharma. Christina E. Høi-Hansen received speaker’s honoraria from Eisai. Caroline Lund received speaker’s honoraria from Eisai, UCB, and Jazz.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

