Estimating the potential impact and diagnostic requirements for SARS-CoV-2 test-and-treat programs
- PMID: 38042923
- PMCID: PMC10693634
- DOI: 10.1038/s41467-023-43769-z
Estimating the potential impact and diagnostic requirements for SARS-CoV-2 test-and-treat programs
Abstract
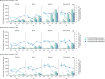
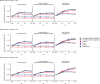
Oral antivirals have the potential to reduce the public health burden of COVID-19. However, now that we have exited the emergency-phase of the COVID-19 pandemic, declining SARS-CoV-2 clinical testing rates (average testing rates = [Formula: see text]10 tests/100,000 people/day in low-and-middle income countries; <100 tests/100,000 people/day in high-income countries; September 2023) make the development of effective test-and-treat programs challenging. We used an agent-based model to investigate how testing rates and strategies affect the use and effectiveness of oral antiviral test-to-treat programs in four country archetypes of different income levels and demographies. We find that in the post-emergency-phase of the pandemic, in countries where low testing rates are driven by limited testing capacity, significant population-level impact of test-and-treat programs can only be achieved by both increasing testing rates and prioritizing individuals with greater risk of severe disease. However, for all countries, significant reductions in severe cases with antivirals are only possible if testing rates were substantially increased with high willingness of people to seek testing. Comparing the potential population-level reductions in severe disease outcomes of test-to-treat programs and vaccination shows that test-and-treat strategies are likely substantially more resource intensive requiring very high levels of testing (≫100 tests/100,000 people/day) and antiviral use suggesting that vaccination should be a higher priority.
© 2023. The Author(s).
Conflict of interest statement
E.H., S.C., B.R., and B.E.N. declare that they are employed by FIND, the global alliance for diagnostics. The remaining authors declare no competing interests.
Figures


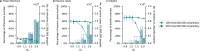
Update of
-
Estimating the potential impact and diagnostic requirements for SARS-CoV-2 test-and-treat programs.medRxiv [Preprint]. 2023 Oct 13:2022.10.05.22280727. doi: 10.1101/2022.10.05.22280727. medRxiv. 2023. Update in: Nat Commun. 2023 Dec 2;14(1):7981. doi: 10.1038/s41467-023-43769-z. PMID: 36238715 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

